Fig. 1. An overview of genetic circuit interactions within and between synthetic minimal cells (synells).
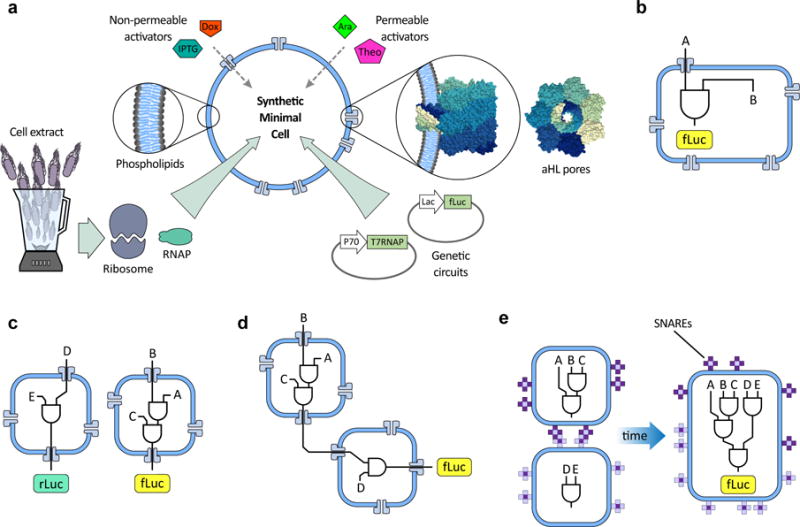
a. Synthetic minimal cells (synells) are semipermeable compartments made from a phospholipid bilayer membrane and various contents. The membrane can display a variety of proteins, including channel-forming proteins such as alpha-hemolysin (aHL). The phospholipid membranes of synells are permeable to molecules such as theophylline (Theo) and arabinose (Ara), and are permeable to others like β-D-1-thiogalactopyranoside (IPTG) and doxycycline (Dox) when aHL channels are present; these molecules can be used for triggering activity within synells. Synells can encapsulate cell lysates with transcriptional and/or translational activity, as well as DNA vectors encoding genes. In this paper, we demonstrate four novel competencies of synells that, together, can be used to create complex, modular genetic circuits. b. We show that synells can contain genetic circuits in which all the components and operations take place within the same liposome. c. We show that two genetic circuits can work independently in separate liposome populations. d. We show that genetic circuits within two different liposome populations can interact. e. We show that genetic circuits can run in parallel in separate compartmentalized reactions; if those reactions are encapsulated by liposomes carrying fusogenic peptides such as SNAREs, the reaction products can be joined together in a hierarchical fashion.
