Abstract
Background
The practice of salvaging recurrent rectal cancer has evolved. Among patients with locally-recurrent disease, we aimed to define their evolving salvage potential over time, and to identify durable determinants of long-term success.
Methods
In consecutive patients undergoing curative-intent multimodality salvage between 1988–2012, predictors of long-term survival were defined by Cox regression analysis and compared over time. Re-recurrence and subsequent treatments were evaluated.
Results
After multi-disciplinary evaluation of 229 patients, curative-intent salvage therapy included preoperative chemotherapy and/or radiation (74%, with 41% undergoing repeat pelvic irradiation), surgical salvage resection with/without intraoperative radiation (83 patients, 36%), followed by postoperative adjuvant chemotherapy (87, 38%). Curative-intent resection involved multi-visceral resection in 47% and bone resection in 30%, leading to a R0 resection rate of 81%. After a median follow-up of 56 months, the 5-year overall survival was 49.8% in 2005–2012, markedly increased from 31.5% in 1988–1997 (p=0.044). Long-term success was associated with R0 resection (p = 0.017) and lack of secondary failure (p=0.003). 125 (55%) patients had re-recurrence at 19 months (median). Repeat operative rescue was feasible in 44% of the patients with local-only and 20% of the patients with distant re-recurrence, and resulted in a median survival of 20 months after re-recurrence.
Conclusions
The long-term salvage potential for recurrent rectal cancer has significantly improved with time, when an individualized treatment algorithm of multimodality treatments and surgical salvage is considered. Durable predictors of long-term success were achieving an R0 resection at salvage operation, avoidance of secondary failure, and feasibility of repeat rescue after re-recurrence.
Introduction
The multimodality treatment of rectal cancer has significantly evolved over the past few decades toward meticulous surgical technique, judicious use of radiation, and modern chemotherapy1, but pelvic recurrence has not been able to be eradicated.2–5 Curative-intent salvage therapy for recurrent rectal cancer has long been conceived 6–10, and multiple studies from specialized centres around the world over the decades have demonstrated that it offers the only potential for cure and for preservation of quality of life (QOL). 11–13
The practice of salvaging recurrent rectal cancer is often reflective of the treatment trends for primary rectal cancer and has likewise evolved over time. The lack of access to specialized centres, concerns about operative complications, acceptance of chemotherapy or radiation as palliation, and the uncertainty about the long-term salvage potential, remain barriers to referring patients for multidisciplinary evaluation and potential salvage. On the other hand, pelvic re-irradiation, advanced peri-operative care, high-resolution pelvic magnetic resonance imaging (MRI) and positron-emission tomography (PET) imaging, and effective modern systemic chemotherapy, have all becomes available in the most recent decade.1, 14, 15 Thus, it is not surprising that the reported outcomes of salvage therapy show significant variability, with median survival of 22 to 60 months, 3-year local control rates ranging between 26–100%, and 5-year distant failure rates of 9–68%.16
Recognizing that patients with locally-recurrent rectal cancer (LRRC) are heterogeneous in their extent of disease, the prior therapies, and underlying tumour biology, we aimed to define the evolving long-term salvage potential of these patients and to identify durable determinants of long-term success over time. We additionally investigated changes in our multidisciplinary practice over time and highlighted key treatment components.
Methods
Management algorithm for LRRC
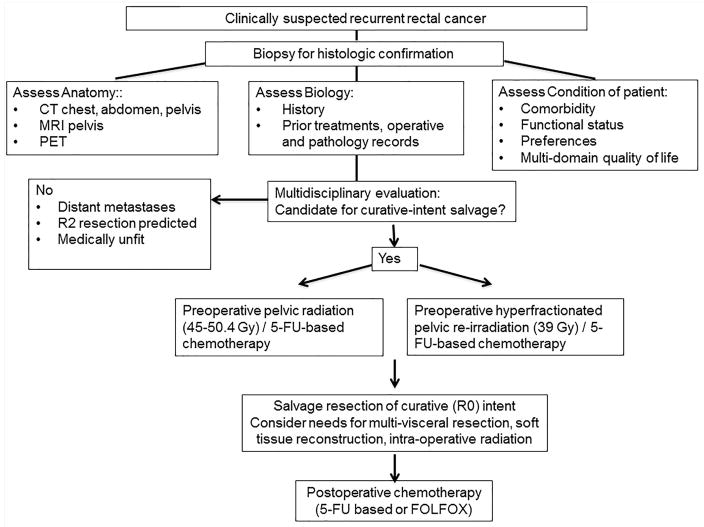
Each patient was evaluated by a multidisciplinary team of colorectal surgical, medical, and radiation oncologists who formulated an individualized treatment plan within a standard framework.(Figure 1) Histologic confirmation of recurrent disease was required in all cases, and tissue biopsy was obtained either endoscopically or percutaneously with image-guidance. The extent of disease is currently evaluated by chest x-ray or computer tomography (CT), abdominal and pelvis CT, MRI of the pelvis, and PET scan. The use of PET had increased after 2001 and became routine since 2005, while the use of high-resolution MRI increased after 2007 and became routine since 2009. The presence of distant metastatic disease and a predicted R2 resection margin12 were generally considered contraindications to curative-intent salvage therapy for locally recurrent disease.
Figure 1.
A management algorithm for recurrent rectal cancer at University of Texas MD Anderson Cancer Centre.
Multimodality therapy typically included preoperative pelvic radiation or re-irradiation with concomitant chemotherapy, salvage surgical resection with curative intent, and postoperative systemic chemotherapy.(Figure 1) Patients who had not received prior pelvic radiation were eligible for preoperative therapy with 50.4 Gy of radiation in 28 fractions with concurrent 5-flurouracil(FU)/Capecitabine; patients who had received prior pelvic radiation were treated with hyperfractionated radiation therapy with a dose of 39gy in 26 fractions of 1.5 Gy twice daily with concurrent Capecitabine (900mg/m2) on days of radiation. Surgical resection was conducted with the planned goal of R0 resection, and multi-specialty surgical teams were assembled for the anticipated need for multi-visceral resection and for soft tissue reconstruction.1 Sphincter preservation is considered whenever possible based on adequacy of rectal margin, freedom from involvement of anal sphincter and levator ani, and anticipated acceptable functional outcomes. Intra-operative radiation (IORT) was administered selectively for patients whose resection margin was negative but close (i.e. ≤ 2mm) or was microscopically positive (R1) based on intraoperative frozen pathology assessment, after a joint consultation between the operative surgeon and the radiation oncologist. Prior to 2001, IORT was typically administered using a linear accelerator delivering 6–15 meV electrons, with dose of 10–20 Gy, prescribed to the 90% isodose line. From 2001, IORT was administered using high dose rate (HDR) iridium-192 brachytherapy using a Harrison-Anderson-Mick (HAM) applicator with a dose of 10–15 Gy prescribed at 1 cm from the radiation source.17 Postoperative adjuvant chemotherapy was used selectively dependent upon prior treatments, surgical pathology, and patient’s performance status postoperatively.
Study cohort
After approval from the Institutional Review Board, consecutive patients with LRRC who underwent multimodality salvage therapy with curative intent at our institution between 1988 and 2012 (inclusively) were identified from the institutional tumour registry. Patients who had gross residual disease (R2) were considered to have not had curative-intent therapy and excluded. Furthermore, patients who had not undergone standard surgery for their primary rectal cancers (for example, local excision or other means of local tumour destruction) were excluded. Patients who had oliogometastatic disease concomitantly resected with curative intent were included.
Patient demographics, tumour characteristics, surgical intervention, and administration of radiation and systemic chemotherapy, were reviewed. Site of pelvic recurrent disease was broadly categorized, based on the predominant disease pattern, as: anastomotic (typically identified endoscopically), mesorectal (involving mesorectal or central presacral soft tissue), lateral (involving lateral pelvic structures), and other. All available operative and pathology reports related to the primary rectal cancers were re-reviewed. Operative reports were specifically reviewed for descriptions of: bowel resection margins, level of vascular ligation, and mesorectal dissection 18–20; pathology reports were reviewed for the College of American Pathologists key elements 21, including histological grade, tumour and nodal evaluation, margin status (distal, proximal, and radial) and distance, and lymphatic and venous invasion. Resection margin was regarded as positive (R1) when any one of the radial, proximal or distal margins were involved by tumour or when tumour-free distance was ≤ 1.0 mm.
The primary endpoints were overall survival (OS), disease-free survival (DFS), and secondary failure or re-recurrence after salvage therapy. All patients were followed from the date of histologic diagnosis of recurrent rectal cancer to the date of their last follow-up or death. Minimum surveillance included clinic visits including laboratory testing every 3–6 months, and annual chest, abdominal and pelvic imaging. Vital status was determined in all patients from medical records, correspondence with patients or local physicians, and/or from the institutional tumour registry. Secondary failure was defined as biopsy-proven re-recurrent disease after multimodality salvage therapy. The first sites of secondary failure were recorded and categorized as local-only if disease were present in the pelvis only, or as distant if metastatic disease in extra-pelvic distant organs were found.
Statistical analyses
Categorical data were described as number and per cent (%), and compared using Χ2 analysis; non-parametric continuous data were described as median, interquartile range (IQR) and compared using the rank sum test. Overall survival (OS) and disease-free survival (DFS) from salvage surgery were estimated using the Kaplan-Meier method. A Cox proportional hazard model was constructed to identify independent predictors of poor OS. To examine the evolution in our practice over time, the study period was divided into three segments, 1988–1996, 1997–2004, and 2005–2012. Compared to the early period of 1988–1996, the middle period was informed by the Dutch TME3 and the German Rectal Cancer2 trials establishing the benefit of total mesorectal excision and neoadjuvant chemoradiation, while modern chemotherapy including oxaliplatin and targeted agents become available since 2005. Differences across time periods were assessed using Cox regression. A two-sided p-value of 0.05 denoted statistical significance. All analyses were conducted using STATA version 11MP (Statacorp, College Station, TX.).
Results
A total of 229 patients met study criteria, including 129 (56%) male patients. The median age at the diagnosis of LRRC was 57 years (IQR: 48–67). Median duration of follow-up was 56 months (IQR: 22–71 months).
Primary rectal cancer treatment
Most patients (n=210, 91.7%) had their primary rectal cancer treated prior to referral to our institution for salvage therapy. The primary procedure had been sphincter-preserving in the majority (n=182, 79.4%). The resection margins (proximal, distal and radial) were negative in 173 (75.5%) patients, and were unknown or positive in the remaining. Despite review of the primary records, pre-treatment clinical stage of the primary rectal tumour was not documented in many cases, but 26.2% of the patients received neoadjuvant chemoradiation. Pathologic stages of the primary cancers are summarized in Table 1.
Table 1.
Details of the treatments for the primary rectal cancers, as well as multimodality salvage therapy for locally recurrent rectal cancer
| N = 229 | (%) | |
|---|---|---|
| Age at diagnosis of recurrent rectal cancer, years (Median, Interquartile range) | 56 | 48–67 |
| Sex | ||
| Male | 129 | 56 |
| Race | ||
| White | 180 | 79 |
| Black or African American | 21 | 9 |
| Hispanic or Latino | 23 | 10 |
| Asian/Other | 5 | 2 |
| Primary rectal cancer | ||
| Surgical procedure | ||
| Sphincter preserving 1 | 182 | 79.5 |
| Non-sphincter preserving 2 | 47 | 20.5 |
| Surgical resection margin | ||
| Negative (R0/R1) | 173 | 75.5 |
| Involved | 17 | 7.5 |
| Incompletely or not reported | 39 | 17.0 |
| Histologic grade | ||
| Well-/Moderately-differentiated | 197 | 86 |
| Poorly /Un-differentiated | 15 | 6.6 |
| Unknown | 17 | 7.4 |
| Lymphovascular and/or perineural invasion | ||
| Yes | 61 | 27 |
| Unknown | 97 | 42 |
| Neoadjuvant chemotherapy and/or radiation | 60 | 26.2 |
| Pathologic stage | ||
| pT1-2N0 | 48 | 20.9 |
| pT3-4N0 | 50 | 21.8 |
| pT1-4N+ | 65 | 28.5 |
| ypT1-2N0 | 7 | 3.1 |
| ypT3-4N0 | 22 | 9.6 |
| ypT1-4N+ | 26 | 11.5 |
| TanyNanyM1 | 3 | 1.2 |
| Unstaged /Unknown | 8 | 3.4 |
| chemotherapy | 134 | 58.5 |
| Multi-modality salvage therapy for recurrent cancer | ||
| Pre-operative chemotherapy and/or radiation | 168 | 73.4 |
| Intraoperative radiation | 83 | 36.2 |
| Post-operative chemotherapy | 87 | 38.0 |
| Salvage Surgery | ||
| Redo LAR/coloanal anastomosis | 46 | 20.1 |
| APR | 55 | 24.1 |
| Pelvic exenteration, anterior | 7 | 3.1 |
| Pelvic exenteration, posterior | 16 | 7.0 |
| Pelvic exenteration, total | 57 | 24.9 |
| Radical resection of pelvic tumor mass | 40 | 17.7 |
| Multi-visceral resection | 108 | 47.2 |
| Bone resection | 68 | 29.7 |
| Oligometastasis resection3 | 10 | 5.7 |
| R0 resection | 183 | 80 |
| Additional LN retrieved at salvage surgery | 104 | 45.4 |
| 30-day or In-hospital outcomes | ||
| Mortality | 2 | 0.8 |
| Morbidity | 116 | 50.6 |
Included low anterior resection (LAR), proctectomy with coloanal anastomosis (coloanal), total proctocolectomy with ileoanal pouch anastomosis (IPAA)
Included abdominal perineal resection (APR), Hartman’s procedure, anterior, posterior, or total pelvic exenteration procedures
Included liver resection (7 patients), and peritoneal/abdominal wall disease resection (3 ptients).
LAR=Low anterior resection
APR=Abdominal perineal resection
LN = lymph nodes
Recurrent rectal cancer diagnosis and treatment
Overall, histologically-proven pelvic recurrence was evident at a median of 27.1 (IQR: 12.1–33.7) months after surgical resection of the primary disease. Most patients were asymptomatic and the diagnosis was triggered by abnormal surveillance imaging (22%), abnormal endoscopy (21%), or elevated carcinoembryonic antigen (CEA,13%). Among symptomatic patients, pain (17%) and anaemia (12%) were most common.
The distribution of recurrent rectal cancer was most commonly central/anastomotic (99 patients, 43.2%), followed by mesorectal/presacral/perineal (83, 36.2%), lateral pelvis (31, 13.6%), and multi-site (16, 7.0%). Oligometastatic disease was present in the liver (3, 1%), para-aortic nodes (2, 1%), and in a periumbilical mass (1, 1%).
The majority of the patients (168 patients, 73.4%) received pre-operative systemic chemotherapy and/or radiation. (Table 1) Fifty-seven (41%) of the 138 patients who received radiation had been previously irradiated for their primary tumours. Patients who had not been treated with prior radiation received 50.4 Gy in 28 fractions. Patients who had received prior pelvic irradiation were treated to a dose of 39Gy in 26 fractions twice daily with concurrent capecitabine at 900mg/m2 on days of radiation. Eighty-seven (38.0%) of the patients received postoperative chemotherapy.
Surgical salvage required multi-visceral resections in approximately half of the patients (47%; Table 1). Bone resection, especially of distal sacrum and coccyx, was required in 30%, while concomitant resection of oligometastatic disease, in 6%. Sphincter preservation was feasible in 20%. Overall, R0 resection was achieved in 185 (81%) patients, while the remaining had a R1 resection. Intraoperative radiation was administered in 83 (36%) patients.
The post-operative mortality rate was 0.9% (Table 1). Two patients expired after total pelvic exenteration, one from respiratory failure and the other from pulmonary embolism. The most common morbidities were wound infection (30 patients; 15%: abdominal 10%, perineal 5%), pelvic abscess (16 patients, 7.0%), urinary tract complications (including urinary tract infection, conduit leaks and ureteral strictures; 15 patients, 7%).
Long-term outcomes of multimodality salvage for LRRC
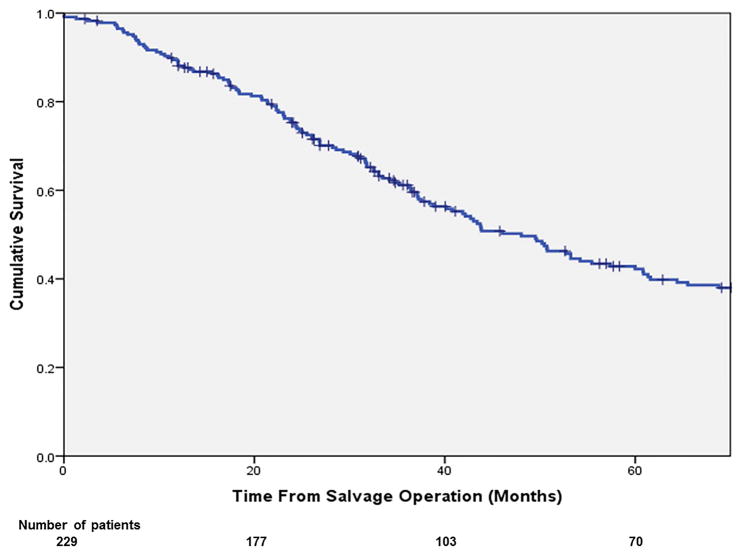
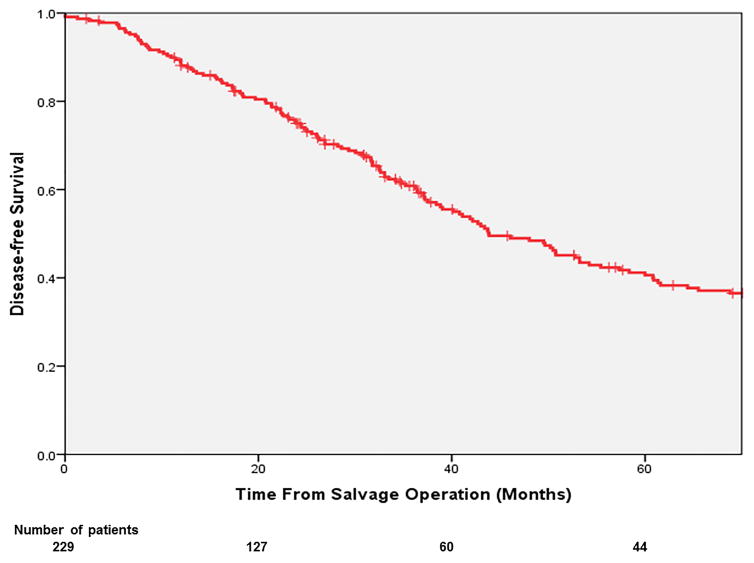
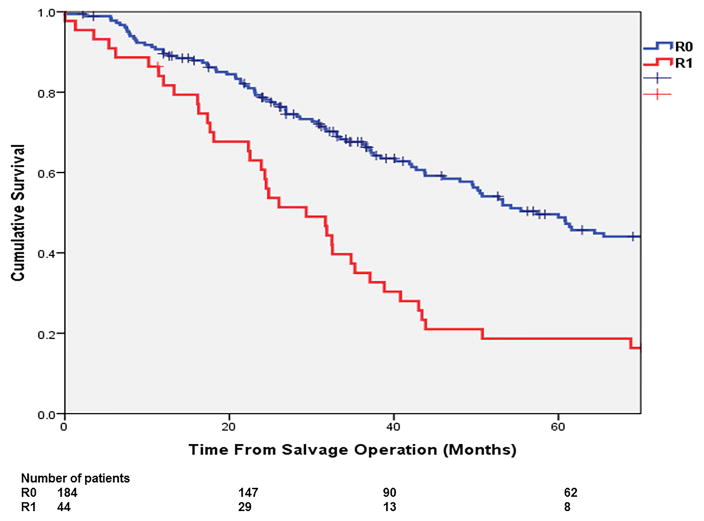
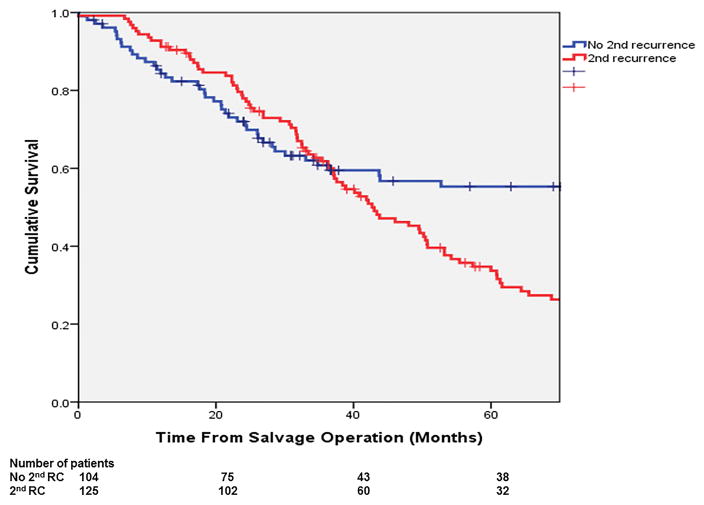
The overall 3- and 5-year OS from salvage surgery were 61.7% and 42.6%. The corresponding 3- and 5-year DSS were 67.0% and 47.3% respectively. (Figure 2a,b) In multivariable analysis (Table 2), independent predictors of poor OS included: non-R0 resection at salvage operation (HR: 1.69; p=0.017) and having a secondary failure (HR: 1.79; p=0.003). The 5-year OS of the patients with a R0 resection was 50%, significantly more favourable than those with a R1 resection (18%; p<0.001; Figure 3a). The 5-year OS of the patients who had developed re-recurrence was significantly inferior to that of those who did not (34.0% vs. 56.0%, p=0.001; Figure 3b).
Figure 2.
Overall survival (a) and disease-free survival (b) of patients after salvage treatment for LRRC.
Table 2.
Multivariable analysis of durable independent predictors of long-term salvage success
| Factor | Hazard ratio for poorer 5-year overall survival | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age (years) | 0.99 | 0.98–1.01 | 0.24 |
| Sex | |||
| Female | 1.0 | ||
| Male | 0.88 | 0.61–1.25 | 0.47 |
| Primary tumour, histologic grade | |||
| Low (grades 1–2) | 1.0 | ||
| High (grades 3–4) | 1.27 | 0.69–2.33 | 0.44 |
| Primary tumour, pathologic stage | |||
| I | 1.0 | ||
| II | 1.40 | 0.83–2.35 | 0.21 |
| III | 1.65 | 0.98–2.77 | 0.061 |
| IV | 1.33 | 0.15–11.47 | 0.78 |
| Primary tumour, radiation treatment | |||
| No | 1.0 | ||
| Yes | 1.15 | 0.72–1.82 | 0.56 |
| Primary tumour, systemic chemotherapy | |||
| No | 1.0 | ||
| Yes | 1.11 | 0.70–1.78 | 0.66 |
| LRRC salvage, type of surgery | |||
| LAR/Coloanal | 1.0 | ||
| APR | 0.84 | 0.46–1.50 | 0.55 |
| Partial/Total exenteration | 1.11 | 0.62–1.91 | 0.77 |
| Radical resection of pelvic mass | 0.92 | 0.48–1.77 | 0.80 |
| LRRC salvage, resection status | |||
| R0 | 1.0 | ||
| Non-R0 | 1.69 | 1.10–2.61 | 0.017 |
| LRRC salvage, additional LN retrieved | |||
| No | 1.0 | ||
| Yes | 0.79 | 0.28–6.37 | 0.72 |
| LRRC salvage, radiation treatment | |||
| No | 1.0 | ||
| Yes | 0.87 | 0.58–1.30 | 0.51 |
| LRRC salvage, systemic chemotherapy | |||
| Yes | 1.0 | ||
| No | 1.41 | 0.91–2.14 | 0.11 |
| Secondary failure | |||
| No | 1.0 | ||
| Yes | 1.79 | 1.22–2.65 | 0.003 |
Figure 3.
The overall survival of the patients (a) with R0 vs. R1 resection (p<0.001); and (b) who developed vs. did not develop a secondary failure (p<0.001).
Secondary failure occurred in 125 (54.6%) patients at 19.4 months (IQR: 7.8–24.9 months) after the index salvage operation. Re-recurrence was loco-regional only in 48 (21.0%), distant only in 58 (25.3%) and both in 19 (8.3%).
Among the 48 patients with local-only re-recurrence, 21 (43.8%) underwent repeat operative salvage of curative intent, including: radical resection of pelvic tumour with en bloc resection (n=10; with distal sacrum/coccyx in 4, entrectomy in 2, seminal vesicle and vaginal cuff in 1 each), partial or total pelvic exenteration (n=8), pelvic regional nodal dissection (n=2), and abdominal perineal resection (n=1). Among the 77 patients with re-recurrence at distant sites, 17 (22.1%) had repeat salvage surgery resecting metastatic disease, in the lung (n=11), liver (n=2), abdominal wall (n=2), retroperitoneum (n=2) and brain (n=1). The remaining patients who were not eligible or declined repeat operative salvage received systemic chemotherapy (n=63), palliative radiation/brachtherapy (n=27), and supportive care (n=8). Patients who underwent repeat salvage surgery for re-recurrence had significantly longer OS than those who were not eligible (median OS of 19.8 vs. 11.3 months; p<0.001).
Evolving oncologic treatments and outcomes over time
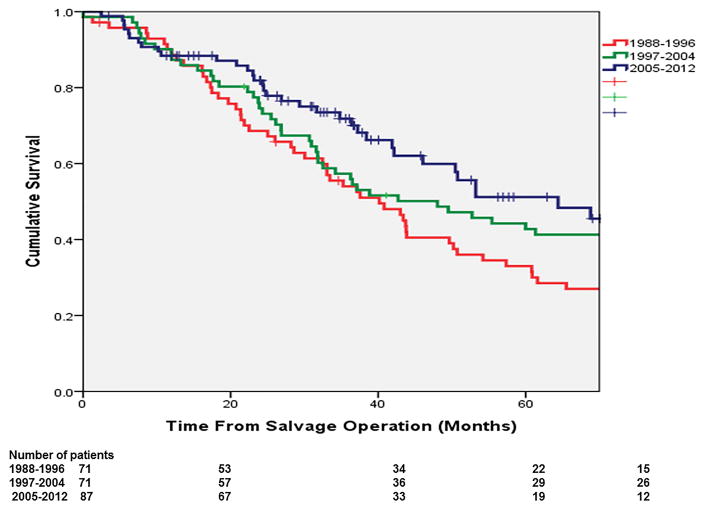
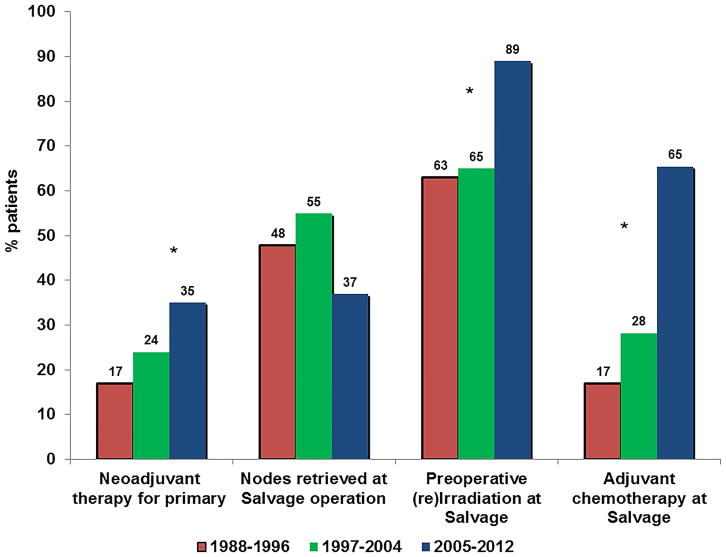
The 5-year OS has significantly improved over our study period. In the most recent time period of 2005–2012, the 5-year OS after salvage was 49.8%, markedly improved since earlier study periods of 1988–1996 and 1997–2004, where the corresponding OS rates were 31.5% and 42.8% (p=0.044; Figure 4). Rates of R0 resection showed an increasing trend from 77% to 84% in association with decreased rates of local-only re-recurrence (24% to 17%), although these differences did not reach statistical significance. The rates of distant failure did not significantly change over time, but repeat rescue surgery has been more frequently performed over time, though the small patient numbers failed to reach statistical significance (p=0.146). The observed improvement in OS was associated with significantly increased use of preoperative therapy for primary rectal cancer (16.9% in 1988–1996 vs. 35.0% in 2005–2012; p=0.0252), a trend toward fewer patients who had residual mesorectal nodes at the time of salvage resection (48% vs. 37%; P=0.102), increased use of pre-operative pelvic (re)irradiation (63% vs. 89%; p<0.001) as well as post-operative chemotherapy in association with salvage surgery (16.9% in 1988–1996 vs. 65.5% in 2005–2012; p<0.001; Figure 5).
Figure 4.
Increased salvage potential of patients over the evolving era of multimodality treatments
Figure 5.
Evolving multimodality treatments for primary and recurrent rectal cancer over time.
Discussion
Despite the heterogeneity of LRRC, careful multidisciplinary planning and often-extensive surgical intervention have been the cornerstones of salvage therapy since its conception. We found that with evolving multi-modality therapy over time, the long-term salvage potential has improved with a 5-year survival 50% currently, representing an absolute 18.3% improvement (49.8% vs 31.5%) over the past two decades. Durable predictors of long-term success were ability to achieve R0 surgical resection and avoidance of re-recurrence/secondary failure, while repeat surgical rescue was feasible and afforded longer survival in eligible patients with re-recurrence.
The most durable determinant of long-term survival over time was achievement of R0-surgical resection. The R0 resection rate of 81% herein compares favourably to rates (26–79%) reported in the literature22–24, and had increased from 77% to 84% in our cohort over time. This success reflects evolved preoperative imaging and careful multidisciplinary surgical planning over time. At our institution, patients are now assessed by contrast-enhanced CT, high-resolution pelvic MRI, and PET. This combination has improved our ability to delineate local and distant disease, and to judge whether R0 surgical salvage is possible or whether R2 resection is predicted. We agree with the consensus recommendation from the Beyond-TME group and have not offered patients with significant metastatic disease or predicted R2 resection curative-intent salvage due to limited evidence of benefit.12, 15 Thus, evolving advanced imaging techniques have helped select the most appropriate candidates for operative salvage while planning for specific pelvic surgical dissection planes. 25, 26 Secondly, to optimize R0 resection, the need for extending beyond the standard TME planes and/or involving multi-visceral or composite bone resection are always anticipated, in accordance with recommendations of the Beyond-TME group.15 As tumour cannot always be differentiated from fibrosis on imaging30,31, other specialty surgeons are involved pre-operatively and intra-operatively as needed. In particular, the need for soft tissue reconstruction is anticipated in these patients with large acquired operative defects in irradiated operative fields. For patients who had undergone a sphincter-preserving procedure, pedicled omental flaps and vascularized rectus muscle flaps were most commonly utilized, while vascularized myocutaneous flaps arising from the rectus, the vastis, the gluteus or the latissimus muscles were utilized for larger perineal wounds especially after multi-visceral resections.27, 28 Indeed, two-thirds of our operations involved multiple surgical specialties and teams of surgeons who have gained experience working with each other have emerged. The significant impact of R0 resection on survival underscores the key role the surgical team plays in the long-term outcome of patients with LRRC.
The re-recurrence or secondary failure after salvage therapy has not been extensively examined in the literature16, but as expected it was another durable predictor of long-term outcome. Secondary failure involving distant sites was more common than local-only failure throughout the study period. Overall, 43.8% of the local-only and 22.1% of the distant re-recurrences were amendable to repeat operative rescue. And this highly selected eligible group enjoyed a median survival of 20 months. Repeat salvage of local re-recurrence followed the same operative principles and mostly involved radical resection of the tumour with en bloc resection of involved structures to R0 resection margin. Our data hinted at rising proportions of patients eligible for repeat operative rescue over time, particularly among those with distant failure. This is likely due to the significant advances in systemic chemotherapy in the recent decade, similar to the increased metastasectomies in liver, lung and peritoneum for patients with stage 4 colorectal cancer.29–32 Thus, increased rescue treatment options for secondary failure, particularly at distant sites, in the future could further extend the overall salvage potential of LRRC.
Salvaging recurrent disease provides a unique reflective lens into the treatment of rectal cancer over time. Compared to the early period of 1988–1996, the middle period (1997–2004) was informed by the Dutch TME3 and the German rectal cancer2 trials, while the most recent period benefited from the availability of modern chemotherapeutic agents, as well as advanced imaging techniques. Indeed, comparing the first and the middle periods, we observed increased use of neoadjuvant therapy, and fewer unresected mesorectal nodes at salvage resection, suggesting increased practice of total mesorectal resection for primary rectal cancer. These changes demanded increasing consideration for (1) pelvic re-irradiation using hyper-fractionated regimen in patients who had received prior radiation for their primary disease17, 33; and for (2) multi-visceral and extensive lateral compartment resections in patients whose recurrent disease no longer represented un-resected mesorectal nodes but disease in the lateral pelvis.34, 35 Although recurrent disease after prior chemoradiation and in the lateral pelvis may be considered more resistant to salvage, these did not significantly influence OS in our multivariate analysis.(Table 2) Comparing the last to prior periods, we observed significantly increased use of pre-salvage chemoradiotherapy and post-salvage “adjuvant” chemotherapy, typically 5FU, oxaliplatin and leucovorin (FOLFOX; 67%), and the inclusion of select LRRC patients with controlled isolated distant metastases. While these changes have been associated with improved salvage potential for LRRC over time, a constant rate of distant failures after salvage therapy remained throughout the study period. This observation suggests that an alternative treatment sequence may be beneficial. We speculate that providing a period of systemic chemotherapy prior to embarking on local salvage surgery may help address the risk for distant failure upfront, decrease the possibility of omitting adjuvant chemotherapy due to postoperative complications, and identify patients with biology that is more favourable for salvage.
Our study is subject to both strengths and limitations. This large series of patients with oncologic follow-up allowed us to examine for durable predictors of long-term outcome and observe changes over time. However, it represents a selected cohort of patients treated at a highly specialized unit. In addition to disease-related factors, the very low perioperative mortality in our study reflects also other patient-related selection factors such as functional status, co-morbidities, and quality of life1, 12. We could not retrospectively reconstruct the larger cohort of all patients with LRRC, and could only estimate, based on prior experience that, only 59% of the patients underwent curative salvage, while 12% underwent non-curative or palliative surgery, and another 28% underwent non-surgical treatment only.12 Furthermore, we did not prospectively capture morbidities related to systemic chemotherapy or any late effects of pelvic radiation. These treatment-associated toxicities should not be ignored as they can linger and impact patient’s survivorship experiences, although patient-reported quality of life after surgical salvage has been acceptable.11, 12, 36 Thus, morbidities associated with multi-modality treatment should be deliberated during overall treatment planning for these complex patients.
Recurrent rectal cancer remains a challenging clinical problem. However, with evolving multi-modality treatments, long-term salvage potential for LRRC has increased over time, with 5-year overall survival now approaching 50%. Durable predictors of success included the ability to achieve R0-resection at salvage operation and avoidance of secondary failure, while repeat surgical rescue of re-recurrences could further extend the overall salvageability. Continued improvements in systemic treatment regimens will be needed to improve the rates of distant failure and further expand the salvageability of these patients.
Acknowledgments
Supported By the University Of Texas MD Anderson Cancer Centre Duncan Family Institute For Cancer Prevention And Risk Assessment Survivorship Research Seed Money Grant (Y.N.Y.), the National Institutes of Health/National Cancer Institute K07-CA133187 (G.J.C.), and the University Of Texas Cancer Centre Core Support Grant (P30CA016672).
The authors would like to thank In Eugene A. Choi, MD, Ja Park, MD, PhD, Amit Agarwal, MD, Sa Nguyen, MS for their assistance in data collection and management.
Footnotes
Presented in part at the American Society of Clinical Oncology Annual Meeting, June 2011.
References
- 1.Rodriguez-Bigas MA, Chang GJ, Skibber JM. Multidisciplinary approach to recurrent/unresectable rectal cancer: how to prepare for the extent of resection. Surg Oncol Clin N Am. 2010;19(4):847–859. doi: 10.1016/j.soc.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. The New England journal of medicine. 2001;345(9):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 4.Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(15):1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 6.Wanebo HJ, Marcove RC. Abdominal sacral resection of locally recurrent rectal cancer. Annals of surgery. 1981;194(4):458–471. doi: 10.1097/00000658-198110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takagi H, Morimoto T, Kato T, Yasue M, Hara S, Suzuki R. Diagnosis and operation for locally recurrent rectal cancer. Journal of surgical oncology. 1985;28(4):290–296. doi: 10.1002/jso.2930280411. [DOI] [PubMed] [Google Scholar]
- 8.Beynon J, Mortensen NJ, Foy DM, Channer JL, Rigby H, Virjee J. The detection and evaluation of locally recurrent rectal cancer with rectal endosonography. Diseases of the colon and rectum. 1989;32(6):509–517. doi: 10.1007/BF02554508. [DOI] [PubMed] [Google Scholar]
- 9.Cohen AM, Minsky BD. Aggressive surgical management of locally advanced primary and recurrent rectal cancer. Current status and future directions. Diseases of the colon and rectum. 1990;33(5):432–438. doi: 10.1007/BF02156274. [DOI] [PubMed] [Google Scholar]
- 10.de Azevedo JP, Dozois RR, Gunderson LL. Locally recurrent rectal cancer: surgical strategy. World journal of surgery. 1992;16(3):490–494. doi: 10.1007/BF02104453. [DOI] [PubMed] [Google Scholar]
- 11.Esnaola NF, Cantor SB, Johnson ML, Mirza AN, Miller AR, Curley SA, et al. Pain and quality of life after treatment in patients with locally recurrent rectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(21):4361–4367. doi: 10.1200/JCO.2002.02.121. [DOI] [PubMed] [Google Scholar]
- 12.You YN, Habiba H, Chang GJ, Rodriguez-bigas MA, Skibber JM. Prognostic value of quality of life and pain in patients with locally recurrent rectal cancer. Annals of surgical oncology. 2010;18(4):989–996. doi: 10.1245/s10434-010-1218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhangu A, Ali SM, Cunningham D, Brown G, Tekkis P. Comparison of long-term survival outcome of operative vs nonoperative management of recurrent rectal cancer. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2013;15(2):156–163. doi: 10.1111/j.1463-1318.2012.03123.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeo HL, Paty PB. Management of recurrent rectal cancer: practical insights in planning and surgical intervention. Journal of surgical oncology. 2014;109(1):47–52. doi: 10.1002/jso.23457. [DOI] [PubMed] [Google Scholar]
- 15.Beyond TMEC. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. The British journal of surgery. 2013;100(8):1009–1014. doi: 10.1002/bjs.9192. [DOI] [PubMed] [Google Scholar]
- 16.Tanis PJ, Doeksen A, van Lanschot JJ. Intentionally curative treatment of locally recurrent rectal cancer: a systematic review. Can J Surg. 2013;56(2):135–144. doi: 10.1503/cjs.025911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyngstrom JR, Tzeng CW, Beddar S, Das P, Krishnan S, Delclos ME, et al. Intraoperative radiation therapy for locally advanced primary and recurrent colorectal cancer: ten-year institutional experience. Journal of surgical oncology. 2014;109(7):652–658. doi: 10.1002/jso.23570. [DOI] [PubMed] [Google Scholar]
- 18.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93(8):583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 19.Smith AJ, Driman DK, Spithoff K, Hunter A, McLeod RS, Simunovic M, et al. Guideline for optimization of colorectal cancer surgery and pathology. Journal of surgical oncology. 2010;101(1):5–12. doi: 10.1002/jso.21395. [DOI] [PubMed] [Google Scholar]
- 20.Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133(10):1539–1551. doi: 10.5858/133.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. [Accessed April 2013];College of American Pathologists Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum. http://www.cap.org.
- 22.Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2009 doi: 10.1111/j.1463-1318.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 23.Kusters M, Dresen RC, Martijn H, Nieuwenhuijzen GA, van de Velde CJ, van den Berg HA, et al. Radicality of resection and survival after multimodality treatment is influenced by subsite of locally recurrent rectal cancer. International journal of radiation oncology, biology, physics. 2009;75(5):1444–1449. doi: 10.1016/j.ijrobp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Caricato M, Borzomati D, Ausania F, Valeri S, Rosignoli A, Coppola R. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2006;32(2):126–132. doi: 10.1016/j.ejso.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Dresen RC, Kusters M, Daniels-Gooszen AW, Cappendijk VC, Nieuwenhuijzen GA, Kessels AG, et al. Absence of tumor invasion into pelvic structures in locally recurrent rectal cancer: prediction with preoperative MR imaging. Radiology. 2010;256(1):143–150. doi: 10.1148/radiol.10090725. [DOI] [PubMed] [Google Scholar]
- 26.Messiou C, Chalmers A, Boyle K, Sagar P. Surgery for recurrent rectal carcinoma: The role of preoperative magnetic resonance imaging. Clin Radiol. 2006;61(3):250–258. doi: 10.1016/j.crad.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Butler CE, Gundeslioglu AO, Rodriguez-Bigas MA. Outcomes of immediate vertical rectus abdominis myocutaneous flap reconstruction for irradiated abdominoperineal resection defects. Journal of the American College of Surgeons. 2008;206(4):694–703. doi: 10.1016/j.jamcollsurg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Wong S, Garvey P, Skibber J, Yu P. Reconstruction of pelvic exenteration defects with anterolateral thigh-vastus lateralis muscle flaps. Plastic and reconstructive surgery. 2009;124(4):1177–1185. doi: 10.1097/PRS.0b013e3181b5a40f. [DOI] [PubMed] [Google Scholar]
- 29.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salah S, Watanabe K, Park JS, Addasi A, Park JW, Zabaleta J, et al. Repeated resection of colorectal cancer pulmonary oligometastases: pooled analysis and prognostic assessment. Annals of surgical oncology. 2013;20(6):1955–1961. doi: 10.1245/s10434-012-2860-y. [DOI] [PubMed] [Google Scholar]
- 31.Warwick R, Page R. Resection of pulmonary metastases from colorectal carcinoma. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007;33(Suppl 2):S59–63. doi: 10.1016/j.ejso.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Weber T, Roitman M, Link KH. Current status of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Clin Colorectal Cancer. 2012;11(3):167–176. doi: 10.1016/j.clcc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Das P, Delclos ME, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, et al. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. International journal of radiation oncology, biology, physics. 2010;77(1):60–65. doi: 10.1016/j.ijrobp.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 34.Yamada K, Ishizawa T, Niwa K, Chuman Y, Akiba S, Aikou T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. The British journal of surgery. 2001;88(7):988–993. doi: 10.1046/j.0007-1323.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- 35.Park JK, Kim YW, Hur H, Kim NK, Min BS, Sohn SK, et al. Prognostic factors affecting oncologic outcomes in patients with locally recurrent rectal cancer: impact of patterns of pelvic recurrence on curative resection. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2009;394(1):71–77. doi: 10.1007/s00423-008-0391-6. [DOI] [PubMed] [Google Scholar]
- 36.Austin KK, Young JM, Solomon MJ. Quality of life of survivors after pelvic exenteration for rectal cancer. Diseases of the colon and rectum. 2010;53(8):1121–1126. doi: 10.1007/DCR.0b013e3181e10c46. [DOI] [PubMed] [Google Scholar]