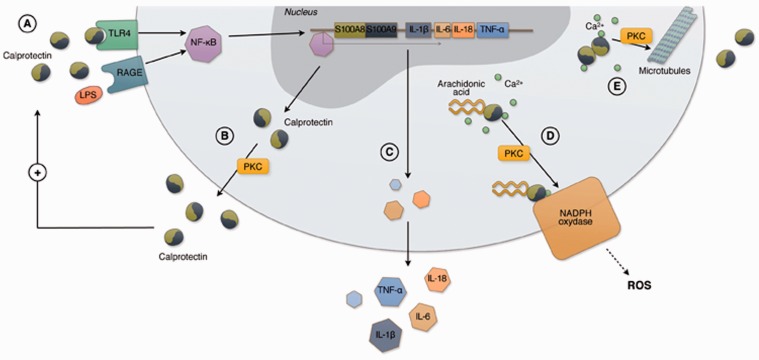
Figure 2.
Intracellular functions of calprotectin in polimorphonucleates and monocytes. CLP is a heterodimer composed of two proteins, S100A8 and S100A9. A danger signal molecule, such as LPS and CLP itself, can bind TLR4 and RAGE directly or through carboxylated glycans triggering inflammation via-NF-ĸB which translocates into the nucleus (a). In the nucleus NF-ĸB induces the expression of further S100A8 and S100A9. CLP is secreted through an energy dependent process, which requires PKC activation (b) and/or the interaction with microtubules (e). TLR4 or RAGE binding by CLP can induce the expression of proinflammatory cytokines and adhesion molecules, such as CD11b and CD18, contributing to the amplification of the inflammatory response and leading to leukocyte adhesion to the endothelium (c). In the presence of calcium, S100A9 subunit binds arachidonic acid and transports it to the NADPH oxidase complex expressed in the plasma membrane with a PKC-dependent mechanism. S100A9 transfers arachidonic acid to gp91phox subunit of the NADPH complex while S100A8 binds to p67phox and rac-2 subunits. Activated NADPH oxidase produces reactive oxygen species which are crucial for the inflammatory activity of granulocytes (d). In the presence of calcium, S100A8 and S100A9 also form heterotetrameters which translocate to the cell membrane and allow tubulin polymerization, microtubules bundling and stabilization of tubulin filaments. CLP regulates the cytoskeleton cell migration (e). (A color version of this figure is available in the online journal.)