Figure 4.
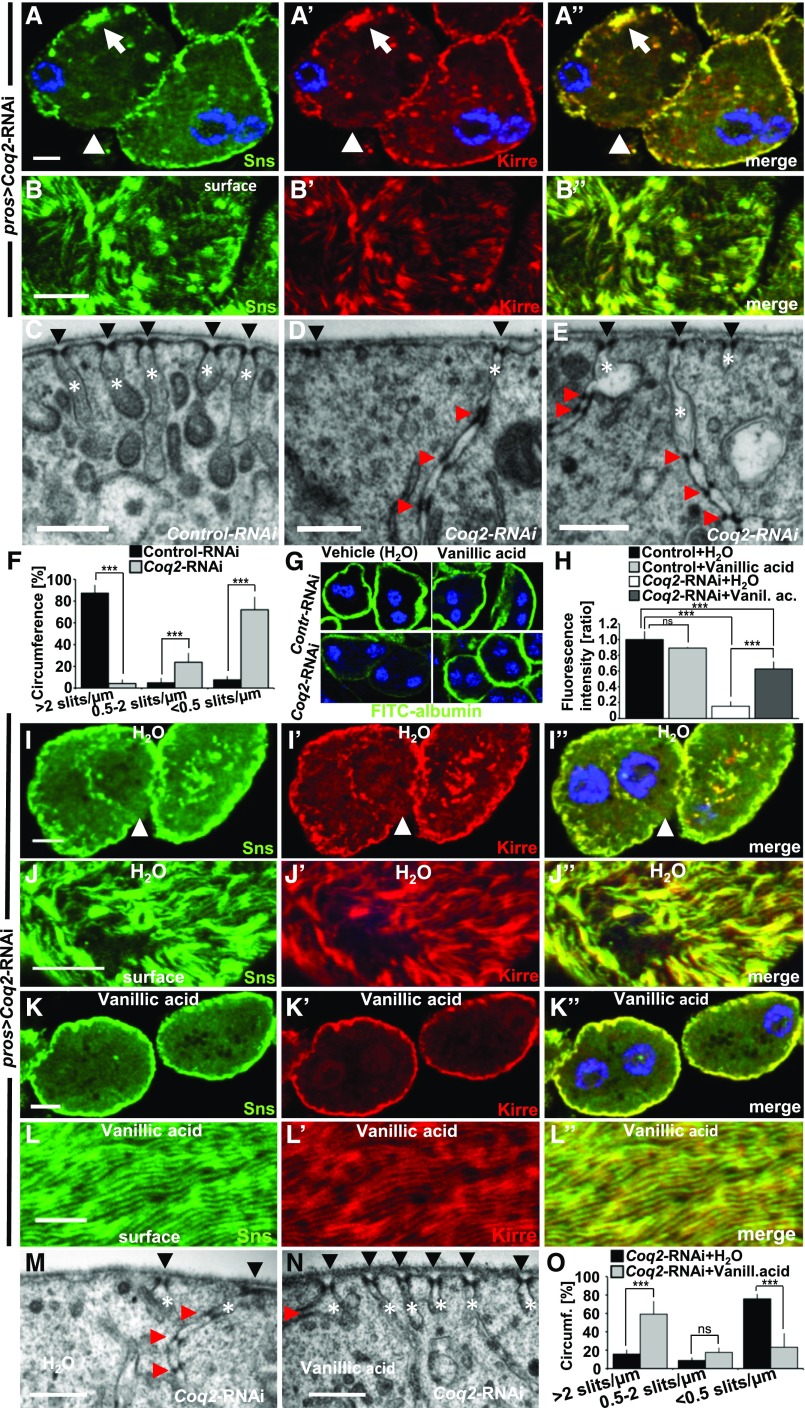
Coq2-RNAi results in mislocalization and loss of slit diaphragm proteins and can be partially rescued by feeding vanillic acid. (A–A”) Garland nephrocytes expressing Coq2-RNAi costained for Sns and Kirre reveal partial protein mislocalization from the slit diaphragm on the cell surface toward the interior of the cell (for comparison see Figure1, A–A’’). The peripheral line of slit diaphragm proteins seems replaced by clustered signals (white arrow) or lost (white arrow head). (B–B’’) Surface section shows a mixture of clustering in puncta, loss, or increased distance of ridges formed by slit diaphragm proteins (for comparison see Figure1, B–B’’). (C) EM image of control-RNAi (GFP) shows slit diaphragms at regular distances (black arrow heads) and labyrinthine channels (white asterisks) that rarely exceed a depth of 500 nm. (D and E) EM images of nephrocytes expressing Coq2-RNAi show reduced number of slit diaphragms (arrow heads) and labyrinthine channels (asterisks). Where present, channels appear elongated and narrower compared with control (C). Slit diaphragms are often mislocalized into the labyrinthine channels and multiplied (red arrow heads). (F) Quantitation of frequency of slit diaphragms measured along one complete GCN diameter for control-RNAi compared with Coq2-RNAi as mean of six cells from three different animals. Frequency of slit diaphragms was classified into three groups: normal (>2 slits/µm), reduced (0.5–2 slits/µm), and sporadic (<0.5 slits/µm). Note the strong reduction of slit diaphragms in Coq2-RNAi. (G and H) Supplementing vanillic acid with the food increases FITC-albumin uptake compared with control treatment with H2O, suggesting a partially restored nephrocyte function. Feeding vanillic acid to flies expressing control-RNAi has no relevant influence on FITC-albumin uptake. (H) Quantitation of experiments from (G). Rescue with vanillic acid blunts the phenotype of Coq2 silencing. n=4 per genotype and intervention. (I–J’’) Nephrocytes from flies treated with vehicle (H2O) show a phenotype similar to untreated cells (A–A’’). (K–L’’) Upon treatment with vanillic acid slit diaphragm proteins are more adherent to cell surface and the fingerprint pattern of Sns/Kirre is restored (L–L’’). (M) EM image of a nephrocyte expressing Coq2-RNAi after treatment with vehicle (H2O) shows a similar phenotype to untreated cells (see D–E). (N) Treating flies expressing Coq2-RNAi with vanillic acid improves frequency of slit diaphragms formed on the surface (black arrow heads). Slit diaphragms inside labyrinthine channels (red arrow head) and elongated labyrinthine channels are less frequently observed than with vehicle alone. (O) Quantitation of slit diaphragm frequency of one complete diameter for Coq2-RNAi with vehicle (H2O) compared with Coq2-RNAi treated with vanillic acid as mean of six cells from three different animals. Frequency of slit diaphragms was classified into three groups: normal (>2 slits/µm), reduced (0.5–2 slits/µm), and sporadic (<0.5 slits/µm). Note the increase of slit diaphragms on surface upon application of vanillic acid. All scale bars represent 500 nm in electron microscopy images and 5 µm in confocal images.