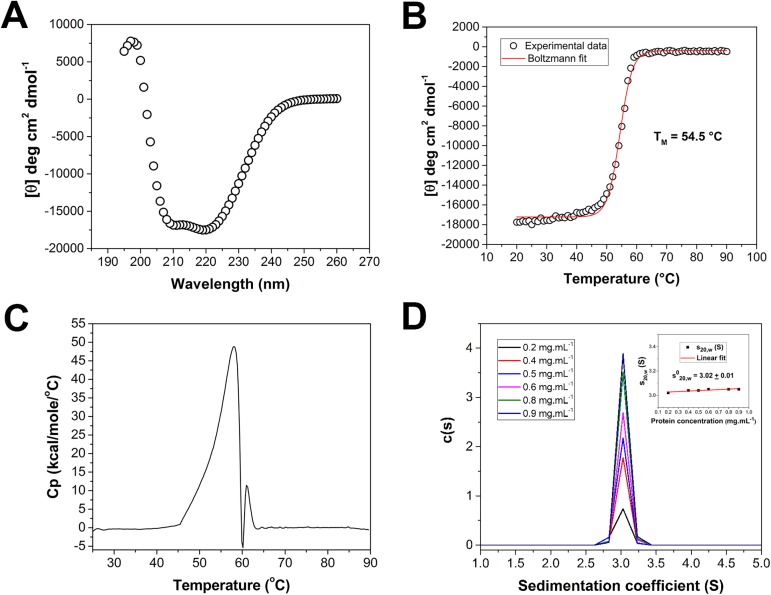
Fig 4. Biophysical characterization.
(A) Circular dichroism spectrum of AfmE1 indicating that the recombinant protein was produced and purified in a folded conformation. CD (B) and DSC (C) thermal unfolding curves showed similar melting temperatures around 55°C. The second peak in the DSC curve corresponds to protein aggregation after denaturation. (D) AUC analysis of AfmE1 at different concentrations confirms that the protein is monomeric in solution with a molecular weight of approximately 39 kDa.