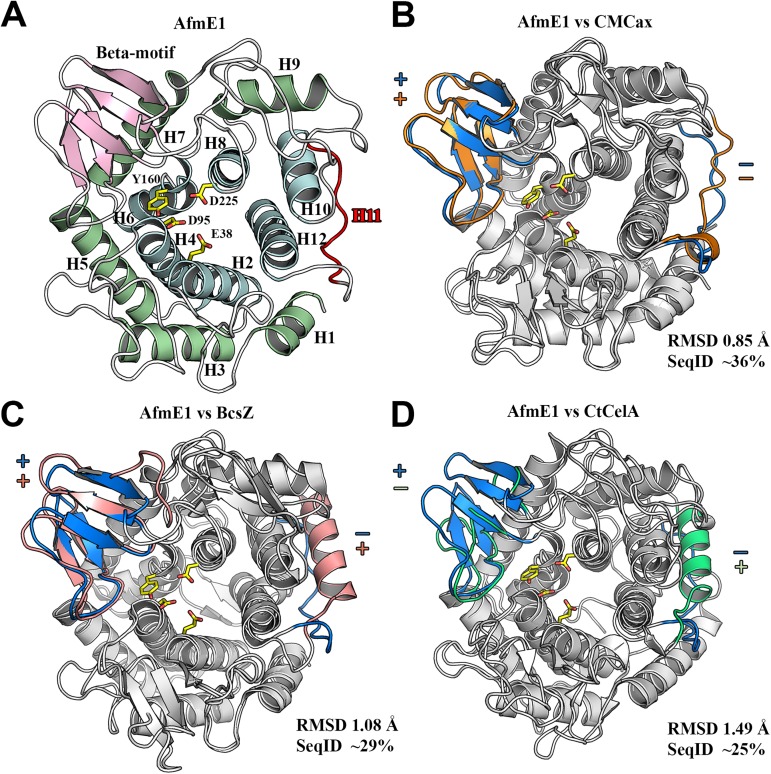
Fig 5. The crystallographic structure of AfmE1.
(A) Cartoon representation of the AfmE1 (α/α)6 barrel fold with the inner and outer helices colored in light-blue and light-green, respectively. The β-motif is shown in pink and the region of the lacking helix-α11 in red. The residues critical for catalysis are depicted as sticks with carbon atoms in yellow. Superposition of AfmE1 structure with those of K. xylinus CMCax (PDB code 1WZZ) (B), E. coli BcsZ (PDB code 3QXQ) (C) and C. thermocellum CelA (PDB code 1CEM) (D). The presence (+) or absence (-) of the β-motif and helix-α11 is indicated and highlighted in blue, orange, pink and green in the structures of AfmE1, CMCax, BcsZ and CelA, respectively. The r.m.s.d. and sequence identity values for each structural alignment are indicated.