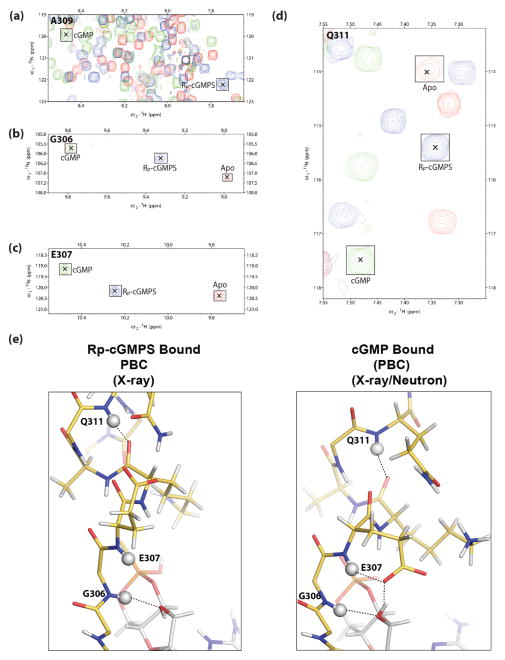
Fig. 3.
NMR confirms the stabilization of a unique PBC conformation by Rp-cGMPS. (a–d), expansions of the overlaid (1H,15N)-HSQC spectra of apo (red contours), Rp-cGMPS-bound (blue contours) and cGMP-bound (green contours) PKG Iβ CNB-B (219–369), illustrating the Rp-GMPS-associated perturbations of key PBC residues: (a), A309, (b), G306; (c), E307; (d), Q311. [24]. For clarity, the peaks are marked with “×” and outlined with labelled squares. Deviations from linearity in the apo vs. Rp-cGMPS vs. cGMP peak positions are likely to arise due to nearest neighbor effects. (e), structures of the PBC of PKG Iβ CNB-B bound to Rp-cGMPS (left) and cGMP (right) (PDB ID: 4KU7). PBC residues (residues 306–313) are shown as sticks complete with protons using the same color scheme as Fig. 1. Protons are colored in white. Backbone amide protons of G305, E307 and Q311 are shown as white spheres. Hydrogen bonding interactions involving these protons are shown as dotted lines.