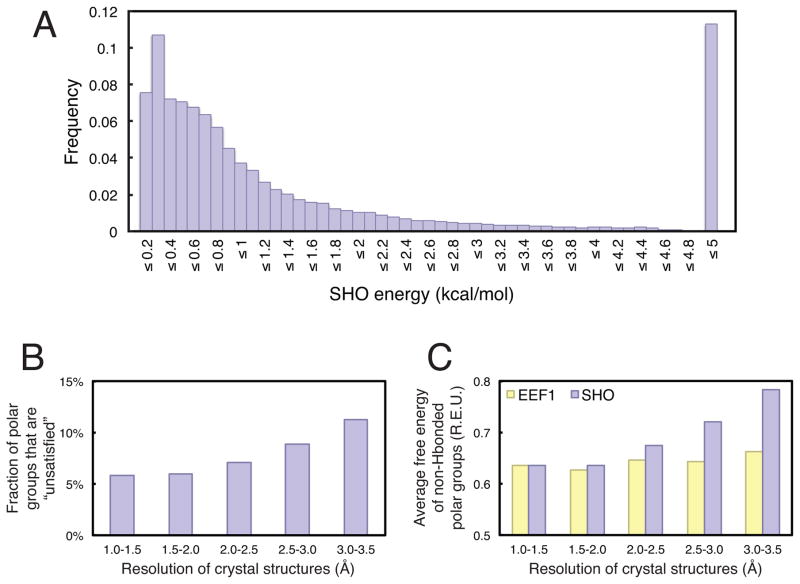
Figure 2. SHO disfavors buried unsatisfied polar groups.
A) The distribution of ESHO values is collected from 207 protein structures with crystallographic resolution 1.0–1.5 Å, for a total of 61,476 polar groups not participating in hydrogen bonds within the solute. Ztot (defined in Equation 2) was set such that the maximum possible value of ESHO would be 5.0 kcal/mol. B) The percentage of polar groups that are not hydrogen bonded to other solute atoms and not “satisfied” by solvent (ESHO > 4.9 kcal/mol) is reported as a function of crystallographic resolution. Higher resolution structures are found to have fewer unsatisfied polar groups. Because most polar groups that meet this criterion are completely desolvated (as seen in panel A), these results are not sensitive to the precise value of ESHO used as cutoff for defining “unsatisfied” groups. C) Drawing only from polar groups that are not hydrogen bonded to other solute atoms, the polar solvation free energy was calculated using SHO and EEF1. While the average polar solvation free energy from SHO increases dramatically at poor resolution, this trend is not observed for EEF1. In both cases, the polar solvation free energies are expressed in Rosetta Energy Units (REUs).