Figure 2. Metabolic pathways of ketone body metabolism in liver and skeletal muscle.
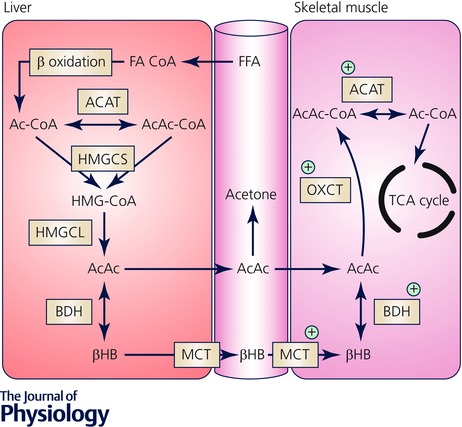
Ketogenesis: FFAs are converted to fatty acyl CoA (FA‐CoA), enter hepatic mitochondria via CPT1‐mediated transport and undergo β‐oxidation to acetyl CoA. Sequential reactions of condensation of Ac‐CoA molecules to acetoacetyl CoA (AcAc‐CoA) by mitochondrial thiolase activity of Ac‐CoA acetyltransferase (ACAT), generation of hydroxymethylglutaryl‐CoA (HMG‐CoA) by hydroxymethylglutaryl CoA synthase (HMGCS), and decomposition of HMG‐CoA, liberating AcAc and Ac‐CoA, in a reaction catalysed by HMG‐CoA lyase (HMGCL). AcAc is the central KB, and some will be exported to the circulation but the majority is reduced to βHB in an NAD+–NADH‐coupled near equilibrium reaction catalysed by BDH, in which the equilibrium constant favours βHB formation. Ketolysis: The only metabolic fate of βHB is inter‐conversion with AcAc, and upon entry into peripheral tissues it is re‐oxidised to AcAc. Covalent activation of AcAc by CoA is catalysed by succinyl‐CoA:3‐oxoacid CoA transferase (OXCT) resulting in generation of AcAc‐CoA. This near equilibrium reaction exchanges CoA between succinate and AcAc, with succinyl‐CoA acting as a CoA donor. Because the free energy released by hydrolysis of AcAc‐CoA is greater than that of succinyl‐CoA, the equilibrium of this reaction thermodynamically favours the formation of AcAc. Two molecules of Ac‐CoA are liberated by thiolytic cleavage of AcAc‐CoA by ACAT, after which Ac‐CoA is incorporated into the TCA cycle. Protein content and enzyme activity that are higher in exercise‐trained skeletal muscle are indicated by the green cross (+).
