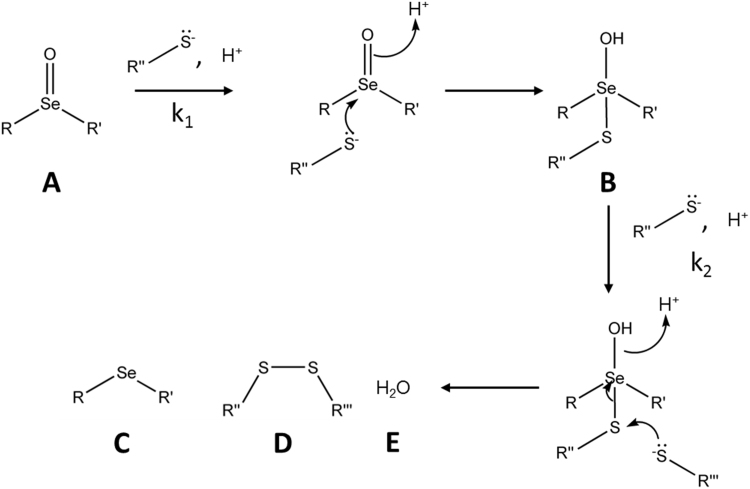
Scheme 1.
Proposed two-step mechanism of selenoxide reduction by thiols. Initial reaction of selenoxides (A) with thiols is proposed to lead to the formation of a seleno-sulfide intermediate (B). The rate constant for the initial reaction is designated as k1. A second thiol then reacts with the intermediate with a rate constant k2, producing a selenoether (C), disulfide (D) and water (E) as products.