Figure 4.
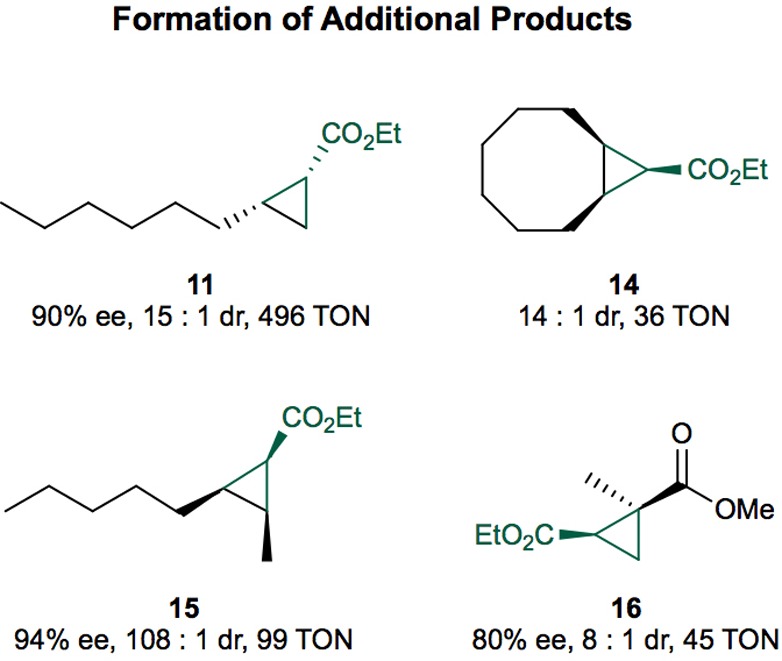
Outcomes of cyclopropanation reactions of additional aliphatic alkenes with EDA. The reactions were conducted on 1 mL scale under the listed conditions using 20 mM alkene and 3 equiv of EDA (added over 3 h by syringe). Product 11 was obtained using the variant CYP119-C317G, A209G (0.02 mol % cat.). Product 14 was obtained using the variant CYP119 C317G, T213G, V254L, L318F, L155W (0.2 mol % cat.). Product 15 was obtained using the variant CYP119(−)-V254L (0.1 mol % cat.), and product 16 was obtained using the variant CYP119(+)-L155W (0.2 mol % cat.). The TON were determined by GC using dodecane as internal standard. The stereochemistry of products was assigned based on NMR analysis and literature reports (see the Supporting Information); the absolute stereochemistry of 16 was assigned based on literature reports (see the Supporting Information); the absolute stereochemistry of products 14 and 16 was not assigned.
