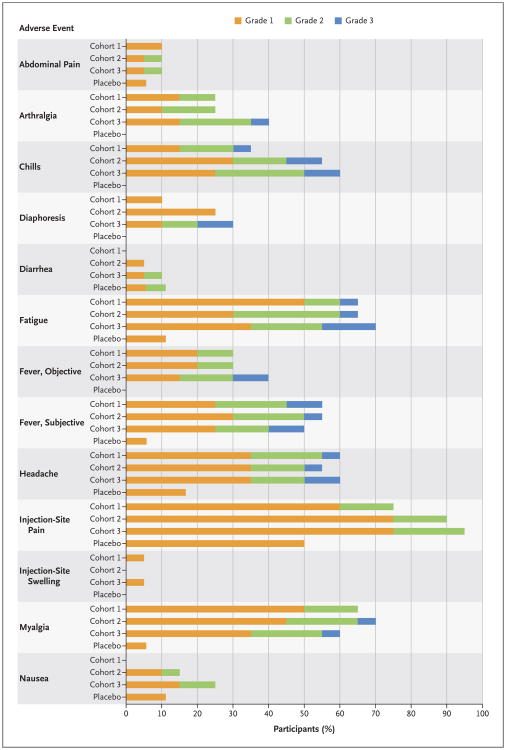
Figure 1. Frequency of Solicited Adverse Events According to Cohort and Grade.
Cohort 1 received a dose of 3 million plaque-forming units (PFU) of the vaccine, Cohort 2 a dose of 20 million PFU, and Cohort 3 a dose of 100 million PFU. All adverse events were assessed for relatedness to the vaccine; events that were judged by the investigating physicians not to be related to the vaccine are not shown. Adverse events were graded for severity on the basis of Food and Drug Administration toxicity grading.15 Unsolicited adverse events and laboratory adverse events are shown in the Supplementary Appendix.