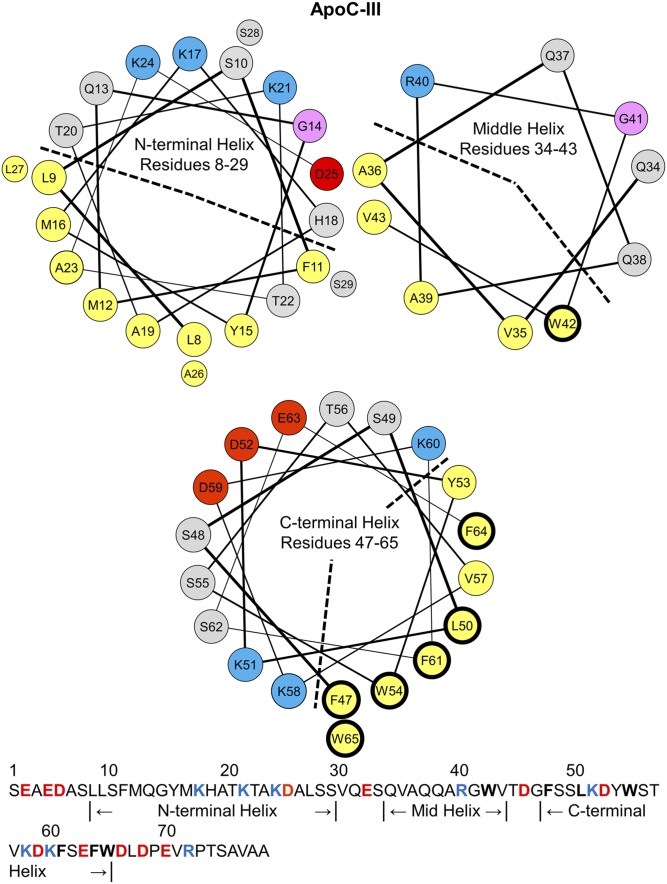
Fig. 1.
Helix wheel diagrams of the N-terminal, middle, and C-terminal α-helices in lipid-bound apoC-III. The residue assignments for the helices in apoC-III were determined by NMR structures of apoC-III:SDS complexes (36). By convention, apolar residues are colored in yellow, polar in gray, basic in blue, acidic in red, and glycine in pink. The N-terminal and middle helices are amphipathic class-G helices, characterized by a random radial distribution of basic and acidic residues. The apolar faces are marked by a dashed line. The C-terminal helix is a class-A amphipathic helix, featuring an apolar face (marked by a dashed line) of eight hydrophobic residues, basic residues at the polar/apolar lipid/water interface, and acidic residues along the polar face. The sequence of apoC-III (79 amino acids) is shown on the bottom. A total of 78–92% of plasma apoC-III is glycosylated at T74 (59, 60), but we used nonglycosylated recombinant apoC-III for this study. Hydrophobic residues W42, F47, L50, W54, F61, F64, and W65 have thick, black outlines in the wheel diagrams and are bolded in the sequence. These residues were mutated to alanines for the studies described here.