Abstract
Murine full-thickness skin transplantation is a well-established in vivo model to study alloimmune response and graft rejection. Despite its limited application to humans, skin transplantation in mice has been widely employed for transplantation research. The procedure is easy to learn and perform, and it does not require delicate microsurgical techniques nor extensive training. Moreover, graft rejection in this model occurs in a very reproducible immunological reaction and is easily monitored by direct inspection and palpation. In addition, secondary skin transplantation with donor-matched or third-party skin grafts can be performed on more complex transplant models as an alternative and uncomplicated method to assess donor-specific tolerance. The complications are low and are in general limited to anesthesia overdose or respiratory distress after the procedure. Graft failure, on the other hand, occurs commonly as a result of poor preparation of the graft, incorrect positioning in the graft bed, or inappropriate placement of the bandage. In this article, we present a protocol for full-thickness skin transplantation in mice and describe the important steps necessary for a successful procedure.
Keywords: Immunology, Issue 119, mouse model, full-thickness skin transplantation, metabolism, rejection, alloimmune, in vivo
Introduction
Organ transplantation is the treatment of choice for patients with end-stage organ failure, and outcomes have improved remarkably with advances in surgical procedures and immunosuppression protocols. However, long-term immunosuppression is associated with significant side effects, and the development of new strategies that promote tolerance remains the goal of modern transplantation research.
Numerous animal models have been developed for basic research in transplantation, to study the mechanisms of allograft rejection and to test immunosuppression approaches for preventing graft rejection and for promoting long-term tolerance1-3. Mouse models have become the mainstay of immunological research due to the exclusive and vast availability of diagnostic and therapeutic antibodies and well-defined inbred and transgenic strains. Skin transplantation is a simple procedure that does not require special microsurgical skills and can be easily monitored postoperatively. Taken together, mouse skin transplantation has been an exceptional tool to study many aspects involved in the alloimmune response, including antigen delivery, cell trafficking, and tissue destruction during graft rejection4,5.
Here, we show the step-by-step procedure for full-thickness skin transplantation using the mouse model, and we describe the important steps necessary for a successful engraftment of the transplanted skin.
Protocol
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institute of Health (NIH) and were approved by the Johns Hopkins University Animal Care and Use Committee (JHUACUC). The specific procedures were performed under the approved ACUC protocols MO13M292 and MO13M370.
1. Donor Skin Harvest
Anesthetize the donor mouse with isoflurane (induction vaporizer at 4%, maintenance at 1 - 2% through the mouse cone). Use the toe pinch withdrawal reflex to monitor the depth of anesthesia.
Using an electric razor, shave the back of the animal and disinfect it with 10% povidone iodine (e.g., Betadine).
Using sterile scissors, gloves, and aseptic technique, harvest donor back skin from the hip to the neck, with blunt dissection at the level of the areolar connective tissue.
Euthanize the animal by cervical dislocation after harvesting the skin graft.
Under a microscope, separate the connective tissue, fat tissue, and panniculus carnosus from the back skin using fine tenotomy scissors. The panniculus carnosus is a thin, transparent muscle responsible for skin twitching movements.
Using sterile instruments technique, cut out 15-mm x 15-mm grafts from the back skin for a 10-mm x 10-mm to 15-mm x 15-mm graft bed.
Store the grafts on gauzes soaked with sterile phosphate-buffered saline (PBS) in a petri dish on ice. NOTE: 8 - 10 grafts can be obtained from one donor mouse.
2. Recipient Skin Transplant
Anesthetize the recipient mouse with isoflurane (induction vaporizer at 4%, maintenance at 1 - 2% through the mouse cone). Use the toe pinch withdrawal reflex to monitor the depth of anesthesia.
Administer 0.02 mg/kg BW of Buprenorphine for postoperative pain relief.
Shave the side of the back of the animal where the graft will be inserted and disinfect with 10% povidone iodine.
Using scissors, cut a 10-mm x 10-mm to 15-mm x 15-mm square of skin. The defect size should be slightly larger (10%) than the graft. Cut as superficially as possible. Take care to preserve the panniculus carnosus and vessels. The panniculus carnosus can be distinguished from the underlying fascia by its mobility and the blood vessels that run superficial to it.
Position the graft on the graft bed, avoiding folds along the edges.
Place 8 sutures on the corners and on the middle of each edge. For each suture, pass the needle through the graft and then through the panniculus carnosus of the graft bed below the surrounding recipient skin.
Remove the anesthetic mask and let the animal recover partially from anesthesia before applying the adhesive bandage.
Wrap the recipient mouse in an adhesive bandage with folded gauze over the graft. Make the bandage by combining two bandages, cutting the adhesive part of one and placing the two absorbent pads together.
Monitor the mouse closely during recovery to ensure that the bandage is not restricting thorax excursion and breathing. Remove the bandage if the respiratory rate decreases or the animal starts to gasp or breath shallowly.
3. Postoperative Care
Administer enrofloxacin 5 mg/kg after surgery for infection prophylaxis.
Place the transplanted mouse in a clean cage over a microwavable heating pad until it fully recovers from anesthesia.
Observe the mouse for 1 hr postoperatively prior to returning it to the housing facility.
Seven days after surgery, anesthetize the mouse as in Step 2.1. Remove the bandage by cutting only through the ventral side of the bandage.
Observe and palpate the graft on the following day for signs of scabbing, contraction, or hardness. If present, the graft may not have achieved proper vascularization and should be considered a technical failure.
Monitor daily for signs of rejection. Consider grafts rejected when ≥90% of the graft tissue becomes necrotic.
Euthanize the rejected animals and harvest them for analysis.
Representative Results
The placement of the bandage on the recipient mouse is an important step of the procedure. The skin graft is positioned on the recipient trunk, between the shoulder, hip, and spine (Figure 1). The bandage is made with folded gauze and the combination of two plastic adhesive bandages. The recipient mouse is placed with the graft down over the gauze on the center of the bandage. Using two curved micro forceps, the lower end of the bandage is pulled first, and then the top of the bandage is wrapped over the mouse to the abdomen.
In complete mismatch models, full-thickness skin grafts are usually rejected in 8 to 12 days. In minor mismatch models, rejection responses are slower, more variable, and the skin graft appearance is characterized by less distinct changes, such as contraction or loss of hair (Figure 2). Acute graft rejection generally begins with swelling and erythema of the graft. These events are followed by graft desiccation, shrinkage, and scab formation (Figure 2B and C). Depending on the degree of MHC mismatch and the immunosuppression protocol, rejection can occur by a subacute process, marked by subtle changes such as loss of hair, pigmentation, dermal ridges, and graft volume. In these cases, a rejected graft appears shiny, white, and hairless, with uneven edges (Figure 2F)14.
Utilizing this model of skin transplantation, we investigated a novel approach targeting T-cell metabolism to prevent graft rejection15. It is well known that metabolic signaling pathways play critical roles in dictating the outcomes of T-cell responses16. Naive T cells rely on mitochondrial oxidative phosphorylation to generate the energy necessary for basic immune surveillance. However, upon activation, effector T cells metabolically reprogram to aerobic glycolysis and exhibit increased glutamine metabolism16,17. Using Balb/c as donors and C57BL/6 as recipients, we observed that inhibiting glycolysis and oxidative phosphorylation (2DG+metformin) or glutamine metabolism alone (DON) prolonged skin graft survival, but blocking the three pathways simultaneously resulted in significantly-increased graft survival (Figure 3C). Moreover, triple metabolic therapy was more effective than conventional cyclosporine or rapamycin (Figure 3A and B). Permanent acceptance was not achieved, and further strategies, including depletion therapy, costimulation blockade, or newer metabolic therapies, may promote better induction of transplant tolerance. However, the study provided two novel insights: the use of non-specific inhibitors to target selective immune cells with increased metabolic demands, as well as the requirement to block the three metabolic pathways simultaneously to obtain more robust immunosuppression in graft rejection.
Calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors constitute most of the conventional immunosuppressive therapy used in clinical transplantation. Median graft survival rates of Balb/c to C57BL/6 skin transplants treated with cyclosporine and rapamycin were 17 and 16 days, respectively. In comparison, recipients treated with metabolic inhibitors that inhibit T cell glycolysis, mitochondrial oxidative phosphorylation, and glutamine metabolism had prolonged survival: the median graft survival was 29 days. Furthermore, the histology of skin grafts from the metabolic therapy group exhibited more intact tissue alignment and less lymphocytic inflammatory infiltrate (Figure 4).
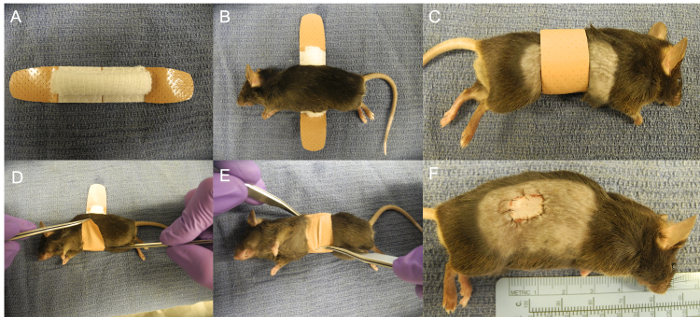 Figure 1:Covering of the Graft with the Bandage. (A) Skin graft placed on the right trunk. (B) Bandage made with a folded gauze and two plastic strips bandages. (C) Recipient mouse placed on the gauze. (D-F) Bandage wrapped around the mouse. Please click here to view a larger version of this figure.
Figure 1:Covering of the Graft with the Bandage. (A) Skin graft placed on the right trunk. (B) Bandage made with a folded gauze and two plastic strips bandages. (C) Recipient mouse placed on the gauze. (D-F) Bandage wrapped around the mouse. Please click here to view a larger version of this figure.
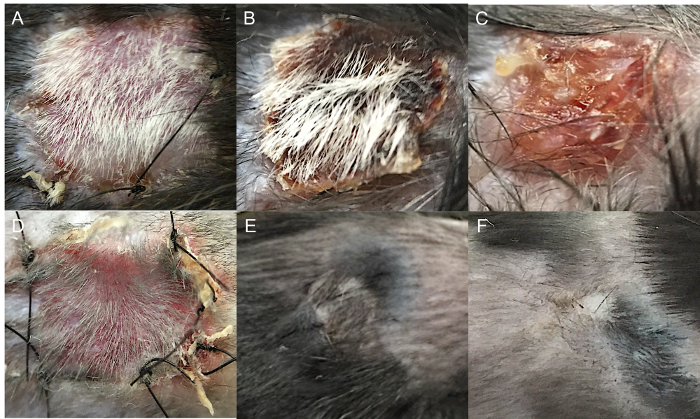 Figure 2:Skin Graft Appearance during Acute and Chronic Rejection. (A) Balb/c to C57BL/6 (complete mismatch) full-thickness skin transplantation without evidence of rejection (day 8). (B) Balb/c to C57BL/6 full-thickness skin transplantation with 50% graft rejection (day 13). (C) Balb/c to C57BL/6 full-thickness skin transplantation with complete graft rejection (day 18). (D) Syngeneic full-thickness skin transplantation (day 8). (E) Syngeneic full-thickness skin transplantation (day 30). (F) C57BL/10 to C57BL/6 (minor mismatch) full-thickness skin transplantation with chronic graft rejection (day 100). Please click here to view a larger version of this figure.
Figure 2:Skin Graft Appearance during Acute and Chronic Rejection. (A) Balb/c to C57BL/6 (complete mismatch) full-thickness skin transplantation without evidence of rejection (day 8). (B) Balb/c to C57BL/6 full-thickness skin transplantation with 50% graft rejection (day 13). (C) Balb/c to C57BL/6 full-thickness skin transplantation with complete graft rejection (day 18). (D) Syngeneic full-thickness skin transplantation (day 8). (E) Syngeneic full-thickness skin transplantation (day 30). (F) C57BL/10 to C57BL/6 (minor mismatch) full-thickness skin transplantation with chronic graft rejection (day 100). Please click here to view a larger version of this figure.
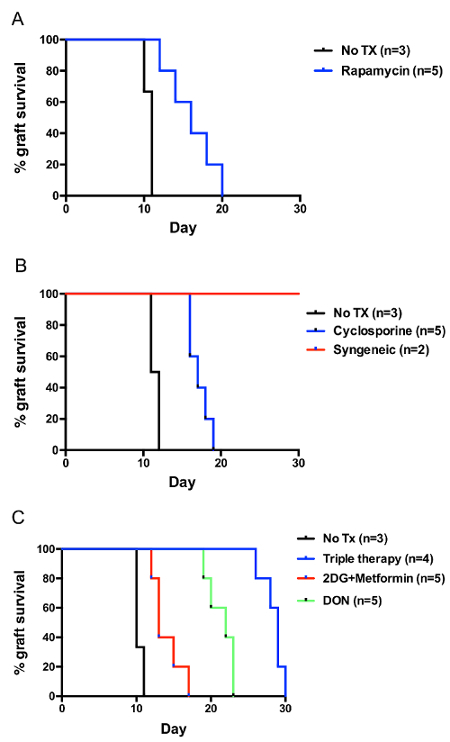 Figure 3:Balb/c to C57BL/6 Skin Graft Survival. (A) Treatment with cyclosporine (25 mg/kg QD). (B) Treatment with rapamycin (3 mg/kg QD). (C) Treatment with metabolic inhibitors, 2-Deoxy-D-glucose (2DG) 500 mg/kg QD, metformin 150 mg/kg QD, and 6-Diazo-5-oxo-L-norleucine (DON) 1.6 mg/kg QOD. All treatments were administered from the day of transplantation until rejection. Please click here to view a larger version of this figure.
Figure 3:Balb/c to C57BL/6 Skin Graft Survival. (A) Treatment with cyclosporine (25 mg/kg QD). (B) Treatment with rapamycin (3 mg/kg QD). (C) Treatment with metabolic inhibitors, 2-Deoxy-D-glucose (2DG) 500 mg/kg QD, metformin 150 mg/kg QD, and 6-Diazo-5-oxo-L-norleucine (DON) 1.6 mg/kg QOD. All treatments were administered from the day of transplantation until rejection. Please click here to view a larger version of this figure.
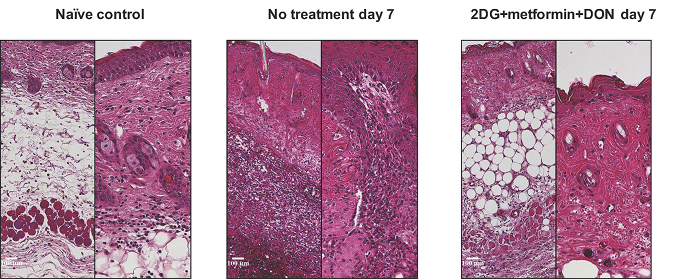 Figure 4:Hematoxylin and Eosin Stain (right, X100; left, X200) of Skin Grafts with Metabolic Therapy at Postoperative Day 7. Please click here to view a larger version of this figure.
Figure 4:Hematoxylin and Eosin Stain (right, X100; left, X200) of Skin Grafts with Metabolic Therapy at Postoperative Day 7. Please click here to view a larger version of this figure.
Discussion
Since its introduction by Medawar, first in human studies and then in rabbits and mice, skin transplantation has been an invaluable model for the study of allogeneic immune responses6,7. In this manuscript, we present a model of large-scale, non-vascularized, full-thickness skin transplantation using the upper and lower back skin. Various alternative methods, including using the tail skin or ear skin of the mouse as the graft tissue source, have been reported to date8,9. These models present the distinct advantage of technical simplicity and allow for high-volume throughput. The relative deficiency of epidermal Langerhans cells and dermal dendritic cells in tail skin grafts, however, leads to delayed rejection as well as to facilitated graft acceptance by the host, particularly in minor MHC mismatch models10,11. Therefore, full-thickness trunk skin grafting is considered a more robust model and more suitable to study T-cell-mediated acute rejection12.
In addition, our model employs simple techniques and does not require advanced microsurgical skills. The operating time is short (~ 10 min per mouse from the onset of anesthesia to the completion of the procedure), and the postoperative morbidity and mortality is negligible. The overall operative mortality (death within 24 hr after surgery plus death before removal of the bandage) has been reported to be 3.8%13. Most of the complications arise from anesthesia overdose or overly-restrictive bandages, which can be easily prevented by close monitoring of respiratory function during the immediate postoperative period and by rapid removal of the bandage at the earliest signs of respiratory distress6,17.
The most critical factor in the process of engraftment is the revascularization of graft tissue by the ingrowth of host vessels to the graft6. The harvested skin requires careful preparation, and the dissection of the panniculus carnosus is a critical step. We prefer to remove the panniculus carnosus under a microscope. It is recommended to start the dissection from one of the edges and to advance slowly by pushing the muscle and separating it from the skin with the blade of a curved tenotomy angled slightly. The site of the graft bed should be far from joints and the spine. Before making incisions into the recipient mouse, the shoulder and hip should be moved to visualize the skin folds that help to demarcate the level of the joints. When removing the recipient skin, it is important to avoid damaging the blood vessels that run superficial to the panniculus carnosus. If bleeding occurs, apply local compression and remove blood and clots that may interfere with engraftment. Finally, the bandage should not cause breathing difficulties that compromise recovery, although it must be sufficiently constrictive to prevent displacement of the graft and auto-mutilation during the process of revascularization. Plastic Strips Band-Aids are adherent and strong enough for this purpose. We combine two bandages to completely wrap the mouse waist and to allow the placement of a folded gauze between the bandage and the graft (Figure 1). The bandage is removed by cutting the ventral side of it, and the rest is removed by the animal, in order to avoid disrupting the graft while trying to remove the bandage manually.
In summary, full-thickness skin transplantation is a well-established model with abundant references in the literature. The procedure is easy to perform, the operating time is short, and the postoperative complications are limited. The critical steps are related to meticulous donor skin harvest and the correct placement of the recipient bandage. Future studies involving biomaterials that promote angiogenesis and tissue repair may further improve the success rate of the procedure.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was funded by NIH grant R01AI077610.
References
- Furtmuller GJ, et al. Orthotopic Hind Limb Transplantation in the Mouse. J Vis Exp. 2016. [DOI] [PMC free article] [PubMed]
- Oh B, et al. A Novel Microsurgical Model for Heterotopic, En Bloc Chest Wall, Thymus, and Heart Transplantation in Mice. J Vis Exp. 2016. [DOI] [PMC free article] [PubMed]
- Oberhuber R, et al. Murine cervical heart transplantation model using a modified cuff technique. J Vis Exp. 2014. p. e50753. [DOI] [PMC free article] [PubMed]
- Jones TR, Shirasugi N, Adams AB, Pearson TC, Larsen CP. Intravital microscopy identifies selectins that regulate T cell traffic into allografts. J Clin Invest. 2003;112:1714–1723. doi: 10.1172/JCI19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli S, Albert ML, Bousso P. Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med. 2011;17:744–749. doi: 10.1038/nm.2376. [DOI] [PubMed] [Google Scholar]
- Medawar PB. The behaviour and fate of skin autografts and skin homografts in rabbits: A report to the War Wounds Committee of the Medical Research Council. J Anat. 1944;78:176–199. [PMC free article] [PubMed] [Google Scholar]
- Billingham RE, Brent L, Medawar PB, Sparrow EM. Quantitative studies on tissue transplantation immunity. I. The survival times of skin homografts exchanged between members of different inbred strains of mice. Proc R Soc Lond B Biol Sci. 1954;143:43–58. doi: 10.1098/rspb.1954.0053. [DOI] [PubMed] [Google Scholar]
- Garrod KR, Cahalan MD. Murine skin transplantation. J Vis Exp. 2008. [DOI] [PMC free article] [PubMed]
- Schmaler M, Broggi MA, Rossi SW. Transplantation of tail skin to study allogeneic CD4 T cell responses in mice. J Vis Exp. 2014. p. e51724. [DOI] [PMC free article] [PubMed]
- Bergstresser PR, Toews GB, Gilliam JN, Streilein JW. Unusual numbers and distribution of Langerhans cells in skin with unique immunologic properties. J Invest Dermatol. 1980;74:312–314. doi: 10.1111/1523-1747.ep12543542. [DOI] [PubMed] [Google Scholar]
- Chen HD, Silvers WK. Influence of Langerhans cells on the survival of H-Y incompatible skin grafts in rats. J Invest Dermatol. 1983;81:20–23. doi: 10.1111/1523-1747.ep12537487. [DOI] [PubMed] [Google Scholar]
- Chong AS, Alegre ML, Miller ML, Fairchild RL. Lessons and limits of mouse models. Cold Spring Harb Perspect Med. 2013;3:a015495. doi: 10.1101/cshperspect.a015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi H, Nomoto K, Good RA. A surgical technique for experimental free skin grafting in mice. Jpn J Surg. 1988;18:548–557. doi: 10.1007/BF02471489. [DOI] [PubMed] [Google Scholar]
- McFarland HI, Rosenberg AS. Skin allograft rejection. Curr Protoc Immunol. 2009;Chapter 4 doi: 10.1002/0471142735.im0404s84. [DOI] [PubMed] [Google Scholar]
- Lee CF, et al. Preventing Allograft Rejection by Targeting Immune Metabolism. Cell reports. 2015;13:760–770. doi: 10.1016/j.celrep.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]


