Abstract
Background
Cognitive dysfunction affects up to 65% of multiple sclerosis (MS) patients and progresses over time. Natalizumab has been shown to be superior to placebo in preserving cognition for the first two years of therapy.
Objectives
The objectives of this study are to understand the impact of natalizumab on cognition beyond two years of therapy and to investigate whether baseline characteristics are predictive of clinical response.
Methods
This is a single-center, 24-month, observational study. Sixty-three patients treated with natalizumab were assessed prior to monthly infusions using a Cogstate battery and the Symbol Digit Modalities Test (SDMT). Patient demographics were collected at baseline. A linear mixed model was conducted with duration of natalizumab therapy as a between-subjects factor (≤2 or >2 years), assessment as a within-subjects factor, and Multiple Sclerosis Severity Score (MSSS) as a covariate.
Results
Aside from the MSSS (p = 0.0074), the two groups were identical. No patient showed evidence of sustained cognitive deterioration over the 24-month period. Baseline parameters including impaired cognition did not influence the trajectory of cognitive change over 24 months.
Conclusions
Our results suggest that natalizumab preserves cognition following four to seven years of continuous therapy. This occurs irrespective of baseline characteristics, including impaired cognition.
Keywords: Cognition, natalizumab, relapsing–remitting, multiple sclerosis
Introduction
Cognitive dysfunction affects up to 65% of patients with multiple sclerosis (MS),1,2 can occur at any stage of the disease regardless of the MS subtype, and can even precede physical disability. Cognitive slowing is detectable in early MS (< 2 years from diagnosis).3 Once present, cognition tends to worsen with time and is unlikely to improve.4 Cognitive dysfunction is most commonly characterized by difficulties with memory, information processing speed and executive skills.4–6 Its presence and severity, however, correlates poorly with physical disability as measured by the Expanded Disability Status Scale (EDSS) and MRI T2 lesion load.7–9 The cognitive reserve hypothesis that posits that intellectual enrichment may protect against neurocognitive decline secondary to disease helps to explain the incomplete relationship between cognitive status and disease in MS.10,11 Predictors of future cognitive deterioration include the presence of cognitive dysfunction and the secondary progressive phase of MS.7 In addition to the underlying disease process, cognitive dysfunction can be caused, or exacerbated, by depression and/or fatigue.12 Cognitive dysfunction can also significantly affect quality of life, resulting in fewer social interactions, higher unemployment,13 and greater difficulties with activities of daily living. Preserving or delaying cognitive decline in MS is therefore an important therapeutic objective.
Many instruments have been proposed to estimate cognition in MS.14 The Symbol Digit Modalities Test (SDMT) is an easy, quick, validated measure of attention, processing speed and working memory. It has shown to be a sensitive measure with robust correlation with brain magnetic resonance imaging (MRI).15 Cognition as measured by the SDMT is a strong predictor of employment status in MS.13 A score of 55 as normal on the SDMT was shown to have a sensitivity of 0.82 and specificity of 0.60.16 The oral version of the SDMT avoids having physical disability as a confounding factor.17 Several versions of the SDMT of equivalent difficulty with test-retest reliability greater than 0.80 have been validated and can be used to decrease practice effect.15 The Cogstate Battery is a computerized battery of simple rapid tests that measures multiple cognitive domains. It is composed of the Detection (DET) (processing speed), Identification (IDN) (attention), One Back (working memory), the Groton Maze Learning Test (GMLT) (executive function), and the International Shopping List Test (ISLT) (verbal learning). It has been used in a number of neurologic and psychiatric diseases, including Alzheimer’s disease and Parkinson’s disease.18
While there have been several studies looking at how disease-modifying drugs reduce the physical symptoms of MS, we are more limited with regards to data on how these medications affect cognition. Of particular interest is whether the effects of certain disease-modifying agents have a beneficial effect on delaying or preventing cognitive impairment in patients with MS over the long term. Natalizumab, in the A Randomized, Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis AFFIRM trial, was one of the first to show benefit on cognition in the short term (two years).19 Natalizumab is a recombinant humanized anti-α4 integrin antibody that blocks the interaction of α4β1 integrin on leukocytes with its counter receptor, vascular cell adhesion molecule-1 (VCAM-1) and mucosal cell adhesion molecule-1 (MAdCAM-1).19 Disruption of these cell adhesion molecule interactions prevents trafficking of mononuclear leukocytes across the endothelium and into the parenchymal tissue. Additionally, a number of other open-label studies have shown, over the short term (<1 year), an immediate benefit in cognition with initiation of natalizumab in MS patients.12 The benefit on relapse rate and progression appears to be sustained in longer-term open-label extension and post-marketing studies such as Safety of TYSABRI Re-dosing and Treatment (STRATA) and Therapy Optimization in Multiple Sclerosis (TOP).20 In clinical practice, however, despite a stable EDSS, patients often complain of cognitive deterioration. Previous studies have shown a weak correlation between physical disability and cognition, although there are no long-term data beyond two years looking specifically at natalizumab treatment and its effect on cognition.
The aim of this study was to evaluate the impact of natalizumab on cognition beyond two years of continuous therapy and to investigate whether baseline characteristics are predictive of clinical response.
Methods
We initiated an open-label, prospective, single-center, observational study.
Sixty-three natalizumab-treated MS patients were recruited from our MS clinic. Informed consent was obtained. The patients were divided into two groups, those with two years or less of natalizumab treatment, and those with more than two years of continuous natalizumab treatment. It was assumed that patients within the two years or less treatment group would behave as previously shown in the AFFIRM trial and thus would act as our reference group. Patients were excluded if they were found to be depressed based on the Beck Depression Inventory (BDI)21 at screening or at any time during the study. Patients were also excluded if they were found to have cognitive decline due to other causes aside from MS.
Patient demographics, MS treatment history, EDSS, Multiple Sclerosis Severity Score (MSSS), education and natalizumab treatment duration were collected at baseline for both groups. The SDMT and a Cogstate battery were performed every four weeks just prior to natalizumab infusion for a period of 24 months. The BDI was administered at baseline and every four months thereafter. Patient data were excluded if the BDI score was greater than 19. The number of patients with no evidence of decline in cognition was calculated at 12 months (interim analysis) and 24 months. Clinically significant decline was defined as a decline in performance of ≥1.96 SD on one or more tests or ≥1 SD on two or more tests that is sustained for at least three months.
In order to minimize practice effects, five different versions of the SDMT were used in five-month cycles. The baseline SDMT score was determined by averaging test scores over the first four months.
A linear mixed model was conducted with duration of natalizumab therapy as a between-subjects factor (≤2 or > 2 years), assessment as a within-subjects factor and MSSS as a covariate.
Results
Sixty-three patients were screened and recruited, and there were no screen failures. The sample was divided into two groups, 34 patients in group 1 (≤2 years of natalizumab treatment) and 28 patients in group 2 (>2 years of treatment).
At baseline, the average duration of natalizumab treatment in group 1 was 0.35 years with a range of 0–2 years. The average duration of natalizumab for group 2 was 3.6 years with a range of 2.1–5.0 years. Except for the MSSS (p = 0.007), there were no statistically significant differences at baseline between the two groups concerning the key demographic variables such as age, education, EDSS, BDI scores, and MS disease duration. The mean BDI at baseline was 7 for both groups. The baseline, mean group SDMT scores were 53 and 60 for groups 1 and 2, respectively. Wide confidence intervals reflect the wide range of individual scores within each group indicative of a cognitively heterogeneous MS population. There was significant overlap between the two groups and thus no real cognitive difference overall between them.
MS disease evolution was similar in both groups as evidenced by a comparable decrease in the group mean EDSS over the 24 months of the study (see Table 1).
Table 1.
The baseline patient characteristics and change in EDSS between the two groups over the 24 months of the study.
| Descriptives split by years on Tysabri |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤2 years |
>2 years |
||||||||
| N | Mean | Std | Miss | N | Mean | Std | Miss | p = | |
| Age | 34 | 43.56 | 10.06 | 0 | 28 | 46.14 | 10.15 | 0 | 0.320 |
| EDSS | 34 | 3.09 | 1.06 | 0 | 28 | 2.84 | 1.48 | 0 | 0.444 |
| EDSS (change) | 25 | –0.34 | 0.62 | 9 | 27 | –0.15 | 0.74 | 1 | 0.321 |
| MSSS | 34 | 4.22 | 2.08 | 0 | 28 | 2.88 | 1.63 | 0 | 0.007 |
| MS duration (years) | 34 | 11.65 | 9.84 | 0 | 28 | 14.46 | 7.73 | 0 | 0.222 |
| Education (years) | 30 | 14.07 | 3.33 | 4 | 27 | 13.67 | 3.49 | 1 | 0.660 |
| BDI | 34 | 7.15 | 4.08 | 0 | 28 | 6.39 | 5.15 | 0 | 0.522 |
EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; MSSS: Multiple Sclerosis Severity Scale; BDI: Beck Depression Index.
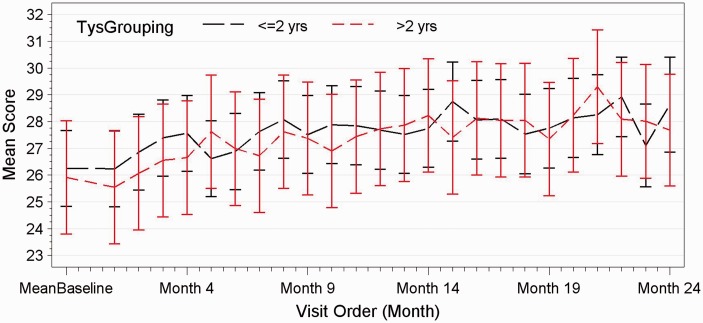
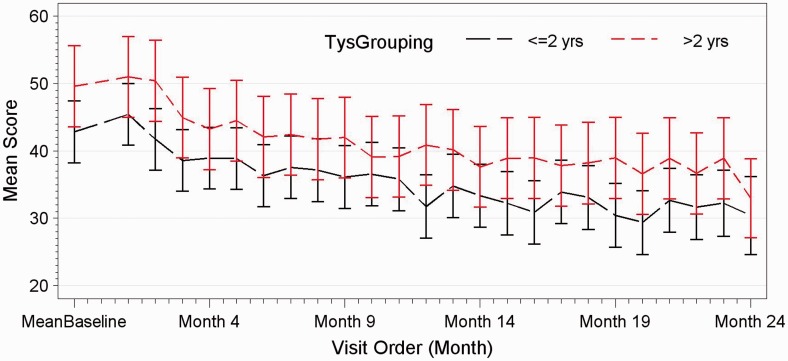
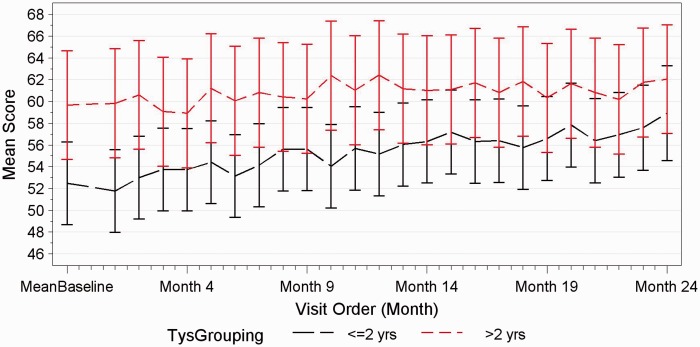
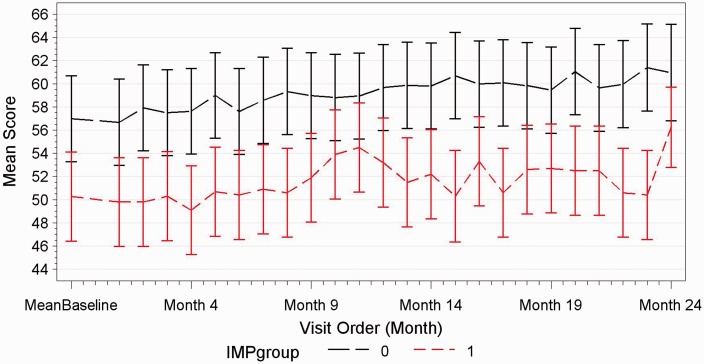
Sixty-two out of 63 patients completed the study. One patient was excluded during the study because of a concomitant depressive episode. Both groups in terms of clinical evolution and cognitive testing behaved exactly the same throughout the 24-month study. No patient in either group showed evidence of sustained cognitive deterioration as per definition over the 24-month period. Irrespective of time on natalizumab, in both groups, significant improvements were observed in group mean scores of executive function (p < 0.0001), verbal memory (p = 0.0012) and working memory (p < 0.0001), whereas processing speed and attention remained unchanged (see Figures 1–3). Baseline characteristics did not influence the trajectory of cognitive change over 24 months. Patients with baseline impaired cognition (SDMT<55) from both groups were compared as a group to non-cognitively impaired patients of both groups (see Figure 4). The impaired group showed greater variability in their scores but overtime behaved as the non-impaired and showed a mild improvement in mean score over the 24-month period.
Figure 1.
The mean score of patients having received less than two years of natalizumab compared to those with more than two years of natalizumab treatment on the International Shopping List Task.
Figure 2.
Change in performance on the Groton Maze Learning Test in those patients having received less than two years of natalizumab versus those with greater than two years of natalizumab treatment.
Figure 3.
Change in performance on the Symbol Digit Modalities Test (SDMT). Performance on the SDMT between patients having received less than two years of natalizumab compared with those with more than two years of natalizumab treatment.
Figure 4.
Change in performance on the Symbol Digit Modalities Test (SDMT) for patients with and without impaired cognition at baseline. Performance on the SDMT between patients with impaired cognition at baseline (red line) and those who were not impaired (gray line).
Discussion
Our study is the first to demonstrate the long-term (two to seven years) impact of natalizumab on cognition in MS patients. Results from the Cogstate battery were consistent with results from the SDMT, providing evidence of the validity of using the SDMT in estimating overall cognition in MS patients.
We believe the difference between the two groups in baseline MSSS could be attributed to the longer duration of natalizumab treatment in group 2. We had no screen failure and only one patient was excluded because of depression throughout the 24-month period.
Despite using five different versions of the SDMT, our monthly testing resulted in significant practice effect as demonstrated by the improvement in group mean scores in executive function, verbal learning and working memory.
Practice effects are typically observed in tests of greater complexity, which would be consistent with the findings in this study, in which improvements were observed on the ISLT, SDMT and GMLT but not the simpler tests of processing speed (DET) and attention (IDN). It is of note that for the latter two, mean group and individual tests scores remained stable, corroborating with the overall thesis of cognitive preservation. It would also suggest that for such tests of higher cognitive function, in a longitudinal analysis over the medium to long term, the persistence of a learning effect should be considered as the normal minimum. It is the authors’ opinion that a practice/learning effect when looked at longitudinally is in itself over the long term an indirect measure of cognitive health. The placebo group in the AFFIRM trial, despite having performed four practice sessions at baseline and only Q3monthly testing during the study, showed a sustained, significant improvement from baseline in Paced Auditory Serial Addition Test (PASAT) scores. The improvement or practice effect was not, however, as marked as in the treated group and began to wear off by the end of the two-year study while the treated group continued to improve. The fact that the improvement/learning effect in our study’s group 2 patients persisted throughout what was for some patients equivalent to seven years of therapy would testify, albeit uncontrolled, to a natalizumab contribution in preserving higher cognitive function, i.e. the ability to learn, in our MS patients over the very long term.
The strongest predictor of future cognitive deterioration is its presence.16 In this study the benefit on cognition did not appear to be influenced by any baseline patient characteristic including pre-existing cognitive impairment. The observation that even cognitively impaired patients at baseline did not further decline in the course of the subsequent two years of the study, we believe, further contributes to the robustness of the results. These findings, however, should be viewed in light of this study being an observational study with a small sample size.
Conclusion
To conclude, cognitive deficits have an integral effect on the quality of life on MS patients. The evolution of cognitive status should be taken into consideration when making therapeutic decisions, especially for patients who already have cognitive deficits. Our study demonstrates that natalizumab appears to preserve the ability to learn and maintain cognition over the long term, even in patients with pre-existing cognitive deficits. We are currently extending this study to collect further data over at least three more years.
Funding
This research was supported by an unrestricted grant from Biogen.
Conflicts of interest.
Dr Jacques has received honoraria from Biogen. B Harel and A Schembri are employees of Cogstate. C Paquette and B Bilodeau are employees of Clinique Neuro-Outaoais. Clinique Neuro-Outaouais has an infusion clinic.
References
- 1.Rao SM, Leo GJ, Bernardin L, et al. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 1991; 41: 685–691. [DOI] [PubMed] [Google Scholar]
- 2.Kujala P, Portin R, Ruutiainen J. The progress of cognitive decline in multiple sclerosis: A controlled 3-year follow-up. Brain 1997; 120(Pt 2): 289–297. [DOI] [PubMed] [Google Scholar]
- 3.Siepman TA, Janssens AC, de Koning I, et al. The role of disability and depression in cognitive functioning within 2 years after multiple sclerosis diagnosis. J Neurol 2008; 255: 910–916. [DOI] [PubMed] [Google Scholar]
- 4.Patti F. Treatment of cognitive impairment in patients with multiple sclerosis. Expert Opin Investig Drugs 2012; 21: 1679–1699. [DOI] [PubMed] [Google Scholar]
- 5.Lovera JF, Kim E, Heriza E, et al. Gingko biloba does not improve cognitive function in MS. Neurology 2012; 79: 1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piras MR, Magnano I, Canu ED, et al. Longitudinal study of cognitive dysfunction in multiple sclerosis: Neuropsychological, neuroradiological, and neurophysiological findings. J Neurol Neurosurg Psychiatry 2003; 74: 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SM. Neurospychology of multiple sclerosis. Curr Opin Neurol 1995; 8: 216–220. [DOI] [PubMed] [Google Scholar]
- 8.Comi G, Filippi M, Martinelli V, et al. Brain MRI correlates of cognitive impairment in primary and secondary multiple sclerosis. J Neurol Neurosurg Psychiatry 1992; 55: 869–876.1431949 [Google Scholar]
- 9.Feinstein A, Ron M, Thompson A. A serial study of psychometric and magnetic resonance imaging changes in multiple sclerosis. Brain 1993; 116: 569–602. [DOI] [PubMed] [Google Scholar]
- 10.Sumowski JF, Wylie GR, Deluca J, et al. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: Functional magnetic resonance imaging evidence for cognitive reserve. Brain 2010; 133(Pt 2): 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedict RH, Morrow SA, Weinstock Guttman B, et al. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsycholo Soc 2010; 16: 829–835. [DOI] [PubMed] [Google Scholar]
- 12.Bagert B, Camplair P, Bourdette D. Cognitive dysfunction in multiple sclerosis: Natural history, pathophysiology and management. CNS Drugs 2002; 16: 445–455. [DOI] [PubMed] [Google Scholar]
- 13.Strober LB, Christodoulou C, Benedict RH, et al. Unemployment in multiple sclerosis: The contribution of personality and disease. Mult Scler 2012; 18: 647–653. [DOI] [PubMed] [Google Scholar]
- 14.Amato MP, Portaccio E, Goretti B, et al. The Rao’s Brief Repeatable Battery and Stroop Test: Normative values with age, education and gender corrections in an Italian population. Mult Scler 2006; 12: 787–793. [DOI] [PubMed] [Google Scholar]
- 15.Benedict RH, Smerbeck A, Parikh R, et al. Reliability and equivalence of alternate forms for the Symbol Digit Modality Test: Implications for multiple sclerosis clinical trials. Mult Scler 2012; 18: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 16.Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modality Test. Mult Scler 2007; 13: 52–57. [DOI] [PubMed] [Google Scholar]
- 17.Berrigan LI, Fisk JD, Walker LA, et al. Reliability of regression-based normative data for the oral Symbol Digit Modalities Test: An evaluation of demographic influences, construct validity, and impairment classification rates in multiple sclerosis samples. Clin Neuropsychol 2014; 28: 281–299. [DOI] [PubMed] [Google Scholar]
- 18.Stochi et al. AFQ056 in parkinson patient with levodopa-induced dyskinesia 13-week, randomized, dose-finding study. Mov Disord 2013. [DOI] [PubMed]
- 19.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor P, Goodman A, Kappos L, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in STRATA MS Study. Neurology 2014; 83: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psych 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]