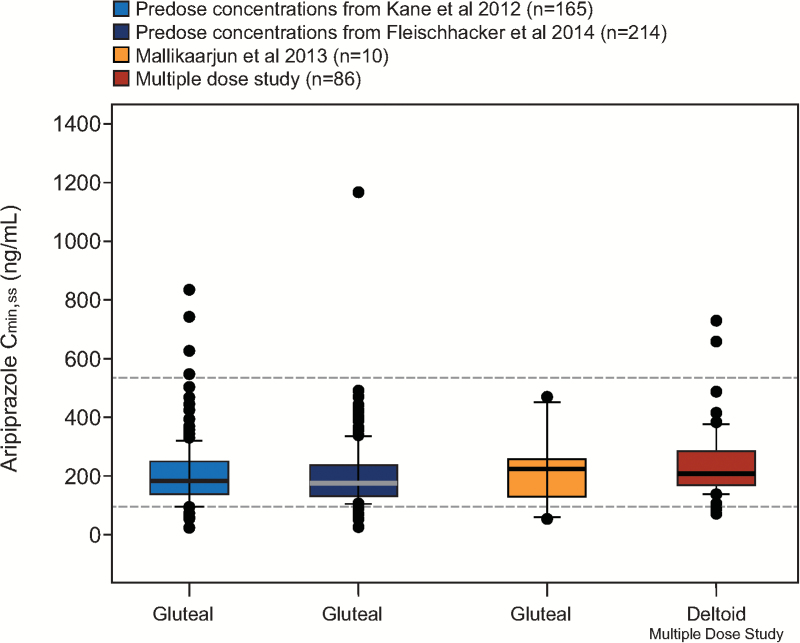
Figure 2.
Aripiprazole Cmin,ss after monthly injections of aripiprazole 400 mg at the deltoid site and comparison with historical data from the gluteal site. Steady-state concentration data for the deltoid site are from the multiple-dose study following the fifth injection, with gluteal/deltoid and deltoid/deltoid administration groups combined. Historical data are from patients with schizophrenia in a phase 3 randomized, placebo-controlled, 52-week study (Kane et al., 2012); a phase 3, randomized, active-controlled 38-week study (Fleischhacker et al., 2014); and a phase 1, open-label, parallel-arm, multiple-dose, 24-week study (Mallikaarjun et al., 2013). Boxes span the 25th and 75th percentiles with a thick horizontal bar at the median. Error bars denote the 10th and 90th percentiles. Dashed horizontal lines represent the therapeutic window (i.e., the median simulated Cmin,ss for oral aripiprazole 10 mg/d [94.0 ng/mL] and the 75th percentile of the simulated Cmax,ss for oral aripiprazole 30 mg/d [534 ng/mL]) based on population pharmacokinetic (PK) simulations of once-daily oral aripiprazole (Raoufinia et al., 2015). Points represent individual outliers below the 10th and above the 90th percentiles. Cmax,ss=maximum plasma concentration at steady state; Cmin,ss=minimum plasma concentration at steady state.