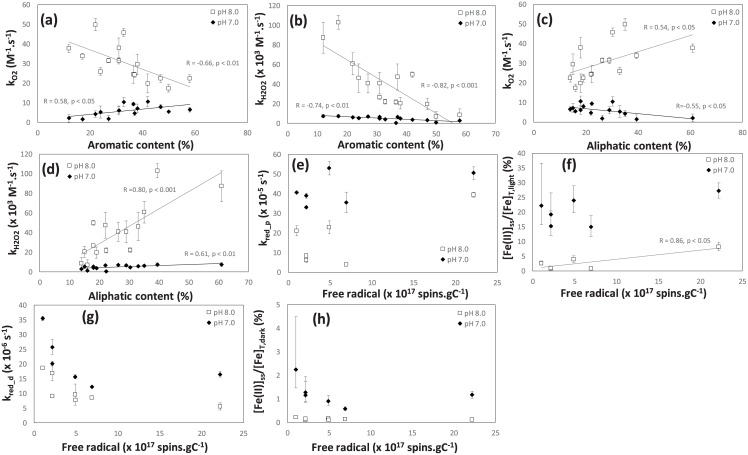
Fig 5. Relationships between major parameters for HS chemical properties (e.g., aromatic and free radical contents) and redox rate constants or steady-state Fe(II) fractions determined in this study: (a) aromatic (in x-axis) vs. kO2 (in y-axis), (b) aromatic vs. kH2O2, (c) aliphatic vs. kO2, (d) aliphatic vs. kH2O2, (e) free radical vs. kred_p, (f) free radical vs. [Fe(II)]ss/[Fe]T, light, (g) free radical vs. kred_d, and (h) free radical vs. [Fe(II)]ss/[Fe]T, dark.
In each figure panel, the data at pH 7.0 and 8.0 were presented. Mean values were indicated by symbols. Error bars for the redox rate constants represent standard deviation. Minimum and maximum values for the steady-state Fe(II) fractions (i.e., [Fe(II)]ss/[Fe]T,dark and [Fe(II)]ss/[Fe]T,light) were represented by upper and lower whiskers, respectively. If significant correlations were observed for the parameters, liner regression lines were inserted with correlation coefficient and p-value.