Figure 2.
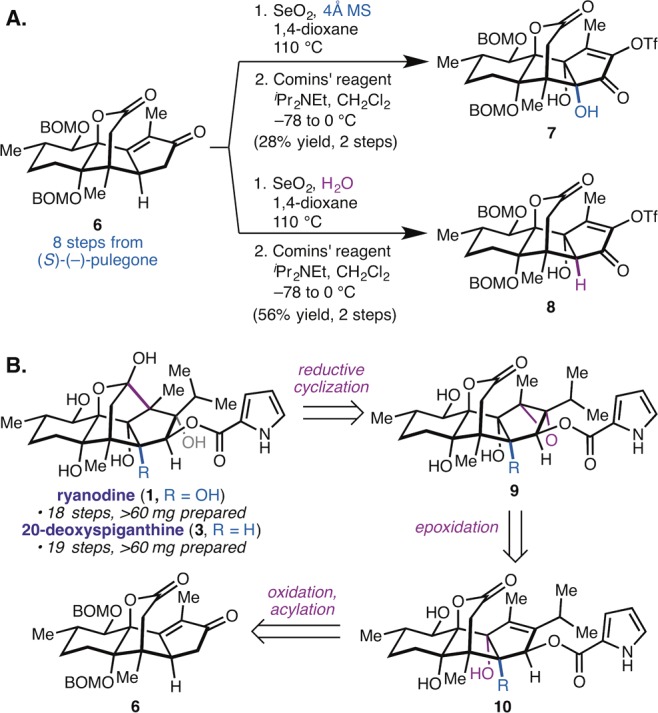
Synthetic considerations. (A) Oxidation products 7 and 8, each accessible from 6 by modification of the SeO2 reaction conditions, can be elaborated to either (+)-ryanodine (1) or (+)-20-deoxyspiganthine (3), respectively. (B) Retrosynthetic analysis for 1 and 3. Key strategic innovation is to directly incorporate pyrrole-2-carboxylate ester (as in 10) prior to reductive cyclization.
