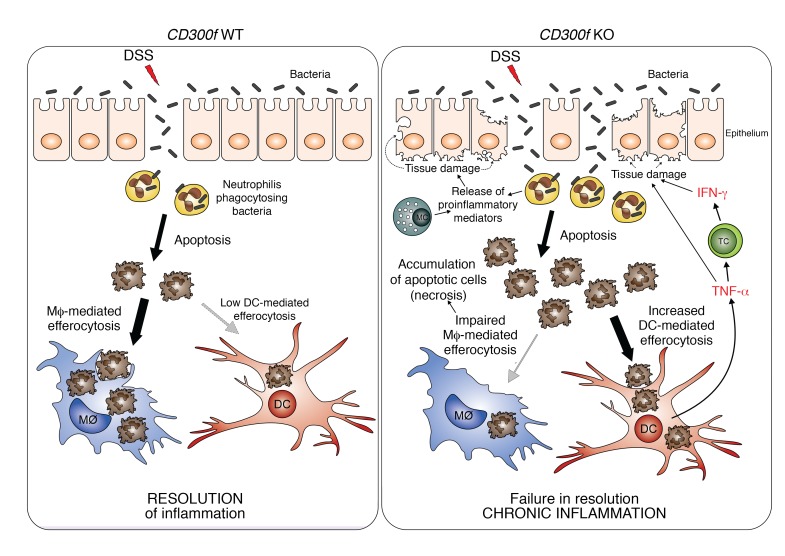
Figure 6. Aggravation of colonic inflammation in the absence of CD300f.
DSS damages intestinal epithelial cells and causes intestine barrier disruption, thereby allowing bacteria to penetrate intestinal barrier. To protect the host from microbial infection, neutrophils are recruited to such inflamed sites, kill the infiltrating bacteria, and undergo apoptosis. In normal conditions (CD300f WT, left), the apoptotic neutrophils are recognized and engulfed by phagocytes (mainly macrophages, Mϕ), which results in resolution of inflammation. However, under CD300f deficiency (CD300f KO, right), macrophages fail to efficiently engulf ACs, leading to accumulation and increased availability of ACs to CD300f-deficient DCs that inherently have increased efferocytic potential. Subsequently, AC-engulfing DCs produce TNF-α, which stimulates T cells (TC) and possibly other immune or nonimmune cells, to produce IFN-γ. Elevated levels of TNF-α and IFN-γ further damage the intestinal barrier, allowing for more bacteria to enter, resulting in prolonged recruitment of neutrophils to fight the microbial intrusion, which further contributes to sustained and elevated apoptosis and accumulation of ACs. In addition, chemoattractants and proinflammatory mediators released by activated neutrophils and mast cells (MC) result in further tissue damage and sustained recruitment of immune cells to the inflamed site, thus aggravating the intestinal inflammation. Eventually, a continuous proinflammatory feedback loop is generated, leading to chronic inflammation. Therefore, CD300f is important for preventing chronic inflammation in the gut by suppressing overactive DC inflammatory responses, and ensuring proper efferocytosis.