Abstract
Background:
This paper presents a new management strategy explaining the process used by implantation of external ventricular drainage (EVD) and very gradual increase of intracranial pressure for treatment of acute hydrocephalus. During the last 30 years’ experience in professional practice, the senior author (M.S.) discovered that there are some options of regulations between cerebral spinal fluid (CSF) production and resorption. This theory shows that minimal continuous increase of the CSF pressure as long as the patient general neurological condition is unchanged and appears as normal can lead to definitive treatment of hydrocephalus without needing to set a shunt. Gradual weaning from EVD is used in some neurosurgical centers related to aneurismal subarachnoid hemorrhage only in a way to treat hydrocephalus in the acute phase, but not as an alternative curative treatment for hydrocephalus, and as far as we know this procedure has not been presented to date in medical literature in this form and this purpose.
Materials and Methods:
Between July 2000 and November 2012, 16 patients suffering from acute secondary hydrocephalus were treated by the method described in the International Neurosciences Institute in Hannover (Germany).
Results:
The causes of hydrocephalus were brain tumors (12), arteriovenous malformations (2), one cavernoma, and one polytrauma. In 11 patients (68.75%), the procedure led to a complete cure and surgical treatment has been excluded after EVD removal without any risk to the patients.
Conclusions:
Minimal gradual increase of CSF pressure by EVD implantation for the treatment of secondary acute communicating hydrocephalus used by senior author as an option is a safe alternative treatment of hydrocephalus and may obviate the need for surgical procedures.
Keywords: Cerebrospinal fluid, external ventricular drainage, hydrocephalus
Introduction
Under normal conditions, cerebral spinal fluid (CSF) is produced at a rate of 0.35 mL/min[1] or 500 mL/day essentially by choroid plexus. It is absorbed at the same time and at the same rate by the arachnoid villi (AV) into the venous circulation. Others absorption pathways have been described.[2,3] Ventricular system is under pressure by resistance to CSF outflow (Rcsf) which intervenes between the sites of secretion and absorption of CSF. CSF pressure (Pcsf) is determined as the result of following formula: Pcsf = Fcsf × Rcsf (Fcsf = CSF flow).
Theoretically, increased Pcsf, indispensable condition of dilatation of the ventricular system and subarachnoid space (hydrocephalus), arises in two circumstances: (1) an increase in CSF secretion and (2) an increase in Rcsf due to a lack of resorption (communicating hydrocephalus) or focal obstacle on CSF pathways (obstructive hydrocephalus). The first condition is controversial. It was suggested in choroid plexus tumors like in papilloma.[4]
The causes of hydrocephalus are numerous. Subarachnoid hemorrhage, meningitis, brain trauma, and neurosurgical operations, especially with opening of the ventricular system are among the most frequent.
The occurrence of hydrocephalus after neurosurgical operations subarachnoid hemorrhage, meningitis, trauma requires in acute phase implantation of external ventricular drainage (EVD) to manage the CSF circulation and to avoid an increase in intracranial pressure. The final treatment in these cases is implantation of ventriculoperitoneal shunt (VPS).
During the last 30 years’ of the senior author's professional practice, he (M.S.) discovered that there are some options of regulations between CSF production and resorption. This theory shows that as long as the patient general neurological condition is unchanged and appears normal, very minimal continuous increase of the CSF pressure can lead to definitive treatment of hydrocephalus. Once the patient tolerates the first step and the secretion of CSF is stabilized or reduced, the pressure increase can be continued every 2–3 days with high pressure until a level that the EVD can be completely closed. Still, the patient for 2–3 days stays in the good and unchanged neurological conditions, and computed tomography (CT) scan demonstrates nonincreasing of the size of ventricular system. In this situation, the EVD is removed, and VPS has been excluded without any risk to the patient.
Through this article, we present this hydrocephalus management discovered by senior author (M.S.) around 30 years ago and used in clinical practice successfully. Gradual weaning from EVD is used in some neurosurgical centers in particular related to aneurysmal subarachnoid hemorrhage[4] in a way to treat hydrocephalus in the acute phase, but not as an alternative curative treatment for hydrocephalus and as far as we know this procedure has not been presented to date in medical literature in this form and this purpose.
Materials and Methods
The study was performed in the International Neurosciences Institute (INI) in Hannover (Germany). We conducted a retrospective chart review of 16 patients treated by the above method between July 2000 and November 2012. These patients presented acute hydrocephalus following a neurosurgical procedure or hemorrhage or trauma (in one case). Before therapeutic decision, all patients received a CT scan which showed hydrocephalus and they underwent EVD implantation. After improving and stability of neurological status of patients, a very gradual and minimal increase of the CSF pressure was affected and continued until they did not need EVD anymore (in 12 cases). Four patients could not tolerate the increase of CSF pressure, so they underwent VPS. In the majority of cases, the patients received a CT scan during the procedure and all of them at the end of procedure.
Results
A total of 16 patients presented an acute hydrocephalus in INI between July 2000, and November 2012 were treated by a minimal gradual increase of CSF pressure (MGIP). All patients needed EVD implantation in acute phase. After an improvement period which lasted on average 48 h, a very gradual and minimal increase of CSF pressure based on each patient conditions (on average 1–2 cm H2O) was performed. This increase was carried out by amplifying the height of EVD bag. Among the causes of hydrocephalus, there were 12 brain tumors, two arteriovenous malformations (AVMs), one cavernoma, and one polytrauma. Patients had the median age of 42.75 (3–79) years with the female/male ratio of 1.67/1 (ten female patients and six male patients). Table 1 shows the characteristics of patients.
Table 1.
Main characteristics of patients
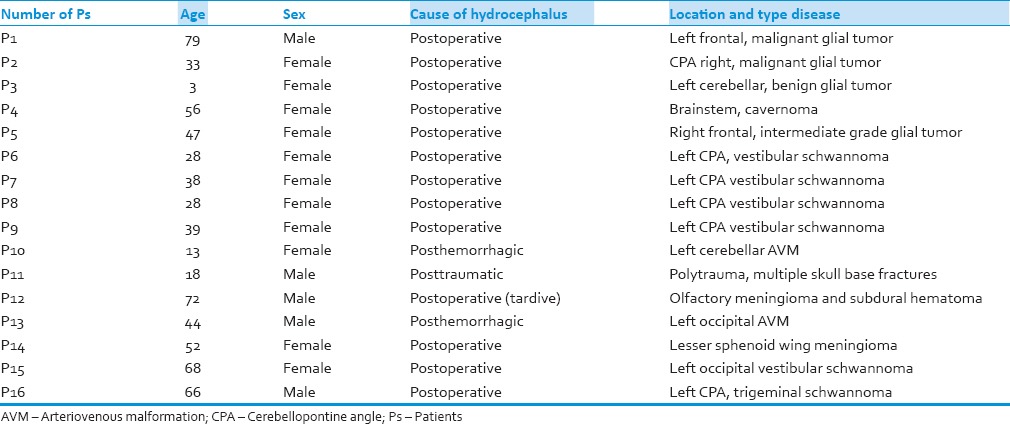
The most common signs and symptoms were headache disturbance of consciousness (drowsiness, temporospatial disorientation, and unconsciousness), headache, nausea, vomiting, and psychomotor slowing. Onset of hydrocephalus was observed and diagnosed in average 4.6 days (1–17). All patients underwent EVD implantation. In all cases of hydrocephaly after EVD implantation, patients improved. EVD remained in place on average for 20.87 days (7–47). In four cases, EVD was changed one or more times (two infections and two EVD dysfunction). In one patient, two EVD were positioned (one in each lateral ventricle). The increase of CSF pressure depended on patients’ tolerance and varied from 3 to 32 days with an average of 15.56 days. Thus, patients had EVD for a median time of 20.87 days (7–47). Eleven patients (68.75%) were weaned successful from the EVD and VPS has been excluded without any risk to them. At the end of the process, all the patients after removal of EVD had no clinical and pathological signs of hydrocephalus. Five patients did not respond to MGIP procedure and underwent VPS. There were the patients with hydrocephalus secondary to two AVM, one lesser sphenoid wing meningioma, one vestibular schwannoma and one, trigeminal schwannoma among them (patients no. 10, 13, 14, 15, and 16). Duration of follow-up varied widely from 6 weeks to 5 years with free shunt patients, and none of them presented signs of hydrocephalus.
Table 2 demonstrates the signs and outcome of patients.
Table 2.
Signs and outcome patients
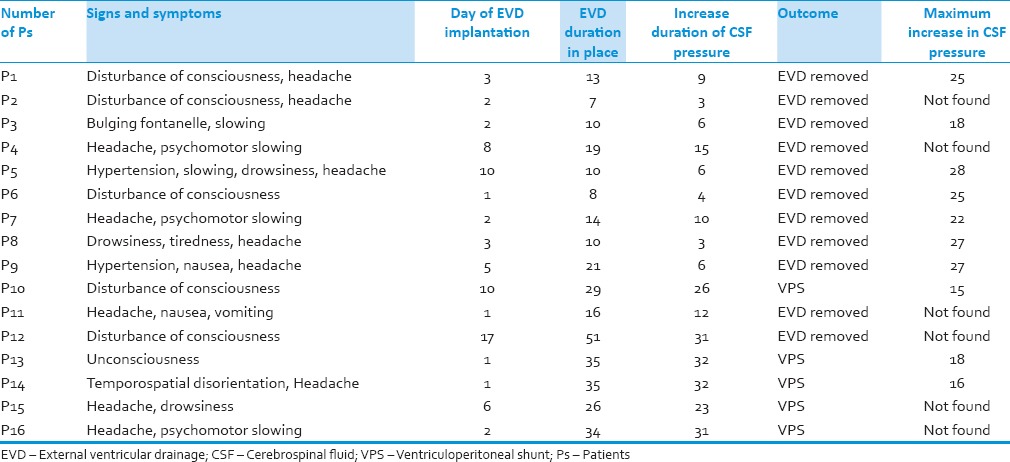
Increase of CSF pressure was performed on average to 24.57 cm H2O in free EVD patients and 17 cm H2O in patients who underwent VPS implantation [Table 2].
Discussion
The acute (symptomatic) communicating hydrocephalus is the main complication in posttraumatic, posthemorrhagic period, and after neurosurgical procedures.[5,6,7,8,9] The rate of hydrocephalus is reported between 10% and 30% in patients who underwent craniotomy[2,3] Duong et al. report that the incidence of postoperative hydrocephalus for skull base surgery is about 8%.[2]
Among 83 patients treated for acute hydrocephalus, Dénes et al. found 52 patients with posttraumatic origin.[10] In a series of 473 patients with subarachnoid hemorrhage, Hasan et al. reported 91 (19%) acute hydrocephalus.[11]
In the acute phase, the treatment of hydrocephalus involves EVD implantation which is a palliative and transient method. In some cases, repeat lumbar punctures can be performed.
VPS is regularly used for curative treatment of hydrocephalus. Alternative treatments are endoscopic third ventriculocisternostomy and lumboperitoneal shunt. The rates of VPS complications are not rare. It is estimated that 50% of VPS malfunction within 2 years will be infected about 10%–15% and ventriculocisternostomy become impermeable within 5 years.
The senior author discovered that there was an alternating option of regulations between CSF production and resorption. That is a very minimal increase of the CSF pressure continuously as long as the patient general neurological condition is unchanged and appears as normal. These conditions can lead to definitive treatment of hydrocephalus. Once the patient tolerates the first step, and the secretion of CSF is stabilized or reduced in 24 h, the pressure increase can be continued every 2–3 days with high pressure until a level that the EVD can be completely closed and still the patient for 2–3 days stays in the good and unchanged neurological conditions, and CT scan demonstrates nonincreasing of the size of ventricular system. In this situation, the EVD is removed, and VPS has been excluded without any risk to the patient. A large number of patients with acute hydrocephalus have been treated in this way in Nordstadt Hospital in Hanover by senior author who had already 30-year experience of this treatment. The purpose of the present paper is to submit this unknown treatment and shows results in patients who were treated by this process in INI in the last 12 years by senior author. Among 16 patients treated in this way, 11 patients (68.75%) showed good response with complete clinical and radiological improvement. The procedure extends the duration of EVD in place but not necessarily the rate of infection and malfunction. We had two infections (12.5%) and two malfunctions EVD (12.5%). These rates are quite close to the rates reported in literature. Five patients (31.25%) did not respond to treatment and underwent VPS. This method as a curative treatment for hydrocephalus spares patients to undergo VPS or other alternating surgical treatments and their short- and long-term complications [Figure 1]. Follow-up period varied from 6 weeks to 5 years with free shunt patients. None of them presented signs of hydrocephalus again. In a comparison between two groups of 81 patients who had aneurysmal subarachnoid hemorrhage and were treated by an EVD, Klopfenstein et al. did not find a significant difference between rapidly weaned group and gradually weaned group, and a total of 51 patients among 81 (61%) required VPS. The first group underwent a rapid weaning of EVD (24 h), and the second group gradually lasted for 6 days.[1] The reason of these results would indicate that the duration of weaning was not long enough. The average EVD duration, in place of 11 patients in our series, in which EVD was removed and VPS not required, was 16.27 days.
Figure 1.
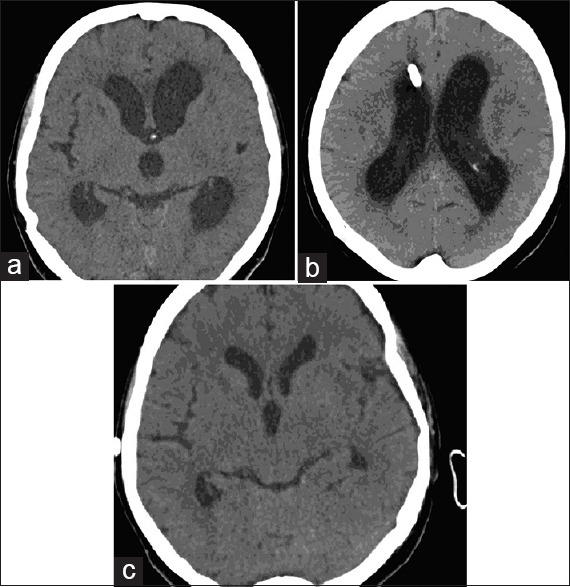
(a) Computed tomography scan shows ventricular dilatation in a patient with acute hydrocephalus in postoperative period, (b) external ventricular drainage in the right ventricle in the same patient, (c) computed tomography scan shows disappearance of ventricular dilatation 3 weeks after external ventricular drainage removal
A pathophysiological explanation would be found in experimental studies in animals in the future.
Davson showed that there was a unidirectional passage of CSF from AV into cranial sinus[12] Marmarou et al. demonstrated that there is a similar absorption in the spine,[13] 25% of CSF drainage is through the spinal AV.[14] Whereas CSF resorption is also carried out by choroid plexus, the arachnoid membrane, the cerebral capillary cranial, and spinal nerves sheaths.[15] The cribriform plate of the ethmoid bone would be an important way of CSF absorption to in the cervical lymph nodes and the cervical lymphatic system.[16,17] These theories were confirmed in particular by Nagra et al. and Rammling et al. studies in animal models.[17,18,19,20] Imaging and microscopic data demonstrated that obstacle to CSF outflow of one of these pathways results in the activation of other pathways.[17,21] In animal model, it was difficult to isolate the lesion pathway responsible and interpretation of capacity of alternate pathways.[20,22] In human with hydrocephalus, it is also difficult to know if a pathological procedure that alters one pathway leaves other pathways intact.[20] Postmortem studies in human and animal models showed some arguments in favor of activating of alternative CSF resorption pathways. Koh et al. and Boulton et al. demonstrated that the CSF absorption in spinal and cranial AV and lymphatic systems influence considerably with increasing CSF pressure.[17,20,21]
Pollay suggests that the fact that hydrocephalus is not frequent after anterior skull base surgery supports the importance of alternative systems in CSF drainage.[20]
Sokolowski argues that CSF absorption is comprised two evacuation mechanisms; a high and a low CSF pressure and in case of failure of a mechanism, the other mechanism revives.[20,21,22,23]
All CSF production and resorption modalities in human brain and spinal cord are based on a regulated entire system, which seems to be flexible and has its own possibility of compensation. Therefore, after having such experiences with gradual increasing of CSF pressure, we can give a chance to the regulation system to change the CSF production and CSF resorption. As a matter of fact that still is a high number of subarachnoid or ventricular hemorrhage, in the majority of cases, a VPS is performed. We should prolong the time of pressure training with EVD in intracranial hemorrhage or other acute hydrocephalus, eventually to reduce the VPS implantation in the future.
Conclusions
In this paper, we present minimal gradual increase of CSF pressure by EVD implantation for a curative treatment of communicating hydrocephalus acute to neurosurgical operations, subarachnoid hemorrhage, or other causes. This procedure is a safe alternative treatment of hydrocephalus and may obviate the need for surgical procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cutler RW, Page L, Galicich J, Watters GV. Formation and absorption of cerebrospinal fluid in man. Brain. 1968;91:707–20. doi: 10.1093/brain/91.4.707. [DOI] [PubMed] [Google Scholar]
- 2.Chazal J, Vaneuville G, Sheye T, Guillot M. Mise au point sur la résorption lymphatique du liquide cérébrospinal. Revue de la littérature. Bull Assoc. Anat. 1989;73:222. [PubMed] [Google Scholar]
- 3.Borgesen SE, Gjerris F. Relationship between intracranial pressure, ventriucalr size and resistance to CSF outflow. J Neurosurg. 1987;67:535–39. doi: 10.3171/jns.1987.67.4.0535. [DOI] [PubMed] [Google Scholar]
- 4.Klopfenstein JD, Kim LJ, Feiz-Erfan I, Hott JS, Goslar P, Zabramski JM, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: A prospective randomized trial. J Neurosurg. 2004;100:225–9. doi: 10.3171/jns.2004.100.2.0225. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg HM, McComb JG, Lorenzo AV. Cerebrospinal fluid overproduction and hydrocephalus associated with choroid plexus papilloma. J Neurosurg. 1974;40:381–5. doi: 10.3171/jns.1974.40.3.0381. [DOI] [PubMed] [Google Scholar]
- 6.Duong DH, O’Malley S, Sekhar LN, Wright DG. Postoperative Hydrocephalus in Cranial Base Surgery. Skull base surgery. 2000;10:197–200. doi: 10.1055/s-2000-9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmarou A, Maset AL, Ward JD, Choi S, Brooks D, Lutz HA, et al. Contribution of CSF and vascular factors to elevation of ICP in severely head-injured patients. J Neurosurg. 1987;66:883–90. doi: 10.3171/jns.1987.66.6.0883. [DOI] [PubMed] [Google Scholar]
- 8.Van Gijn J, Hijdra A, Wijdicks EF, Vermeulen M, Van Crevel H. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1985;63:355–62. doi: 10.3171/jns.1985.63.3.0355. [DOI] [PubMed] [Google Scholar]
- 9.Bontke CF. Medical complications related to traumatic brain injury. Phys Med Rehabil: State Art Rev. 1989;3:43–58. [Google Scholar]
- 10.Dénes Z, Lantos A, Szél I, Thomka M, Vass M, Barsi P. Significance of hydrocephalus following severe brain injury during post-acute rehabilitation. Ideggyogy Sz. 2010;63:397–401. [PubMed] [Google Scholar]
- 11.Hasan D, Vermeulen M, Wijdicks EF, Thomka M, Vass M, Barsi P. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke. 1989;20:747–53. doi: 10.1161/01.str.20.6.747. [DOI] [PubMed] [Google Scholar]
- 12.Davson H. Physiology of the ocular and Cerebrospinal Fluids. London: Churchill; 1956. [Google Scholar]
- 13.Marmarou A, Shulman K, LaMorgese J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg. 1975;43:523–34. doi: 10.3171/jns.1975.43.5.0523. [DOI] [PubMed] [Google Scholar]
- 14.Edsbagge M, Tissel M, Jacobsson L, Wikkelso C. Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1450–5. doi: 10.1152/ajpregu.00215.2004. [DOI] [PubMed] [Google Scholar]
- 15.sakka L, Coll G, Chazal J. Anatomie et physiologie du liquide cérébrospinal. Otorhinolaryngol. 2011;128:359–66. [Google Scholar]
- 16.Davson H, Segal MB. Physiology of the Cerebrospinal Fluid and Blood-Brain Barriers. London: CRC Press; 1996. [Google Scholar]
- 17.Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: Is it time to embrace a new concept of cerebrospinal fluid absorption. Cerebrospinal Fluid Res. 2005;2:6. doi: 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagra G, Li J, McAllister JP, II, Miller J, Wagshul M, Johnston M. Impaired lymphatic cerebrospinal fluid absorption in a rat model of kaolin-induced communicating hydrocephalus. Am J Physiol Regul Integr Comp Physiol. 2008;94:R1752–9. doi: 10.1152/ajpregu.00748.2007. [DOI] [PubMed] [Google Scholar]
- 19.Rammling M, Madan M, Paul L, Behnam B, Pattisapu JV. Evidence for reduced lymphatic CSF absorption in the H-Tx rat hydrocephalus model. Cerebrospinal Fluid Res. 2008;5:15. doi: 10.1186/1743-8454-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael P. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;21:7–9. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulton M, Armstrong D, Flessner M, Hay J, Szalai JP, Johnston M. Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol. 1998;275:R889–96. doi: 10.1152/ajpregu.1998.275.3.R889. [DOI] [PubMed] [Google Scholar]
- 22.Papaiconomou C, Bozanovic-Sosic R, Zakharov A, Johnston M. Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am J Physiol Regul Integr Comp Physiol. 2002;283:R869–76. doi: 10.1152/ajpregu.00173.2002. [DOI] [PubMed] [Google Scholar]
- 23.Sokolowski SJ. Bolus injection test for measurement of cerebrospinal fluid absorption. J Neurol Sci. 1976;28:491–504. doi: 10.1016/0022-510x(76)90120-9. [DOI] [PubMed] [Google Scholar]


