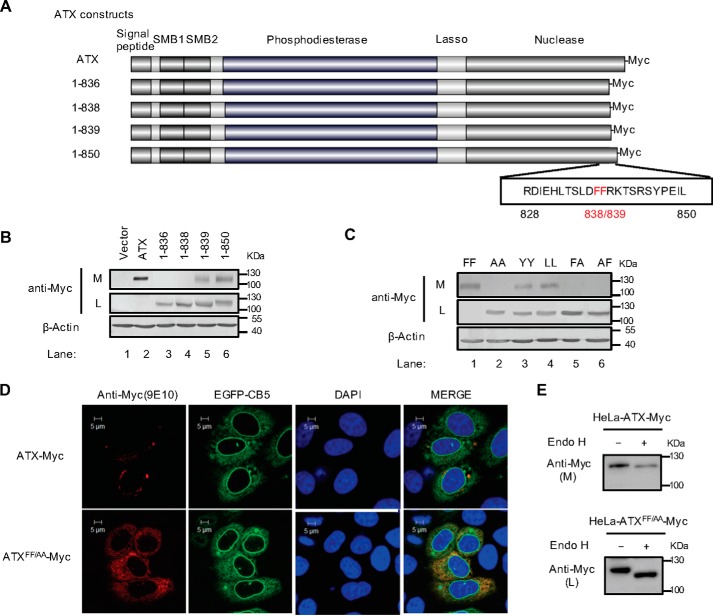
Figure 1.
Identification of a di-phenylalanine (838FF839) motif essential for ATX secretion. A, schematic representation of the wild-type ATX and the truncated ATX mutants with Myc tag at the C terminus. The number of amino acid residues in each ATX protein is indicated, and the di-phenylalanine (838FF839) motif is labeled in red. B, wild-type ATX and the truncated ATX mutants with C-terminal Myc tag were expressed in HeLa cells as indicated. The levels of ATX-Myc or ATX mutant with Myc tag in cell lysates (L) and culture medium (M) were detected by immunoblotting with anti-Myc antibody. C, wild-type ATX (FF) and the indicated ATX mutants, in which the FF motif was replaced by two alanines (AA), two tyrosines (YY), two leucines (LL), or AF, were expressed in HeLa cells as indicated. The levels of ATX-Myc or ATX mutant with Myc tag in cell lysates and culture medium were detected by immunoblotting with anti-Myc antibody. D, plasmid pcDNA3-ATX-Myc or pcDNA3-ATXFF/AA-Myc, in which the FF motif was replaced by AA, was co-transfected with pEGFP-N1-CB5 into HeLa cells. Cells were fixed and permeabilized 48 h after transfection. ATX-Myc and ATXFF/AA-Myc were visualized by confocal microscopy with anti-Myc (9E10) monoclonal antibody (red). Endoplasmic reticulum was labeled by EGFP-CB5 (green). Nuclei were counterstained with DAPI (blue). E, Endo H treatment of secreted ATX-Myc and intracellular ATXFF/AA-Myc. The concentrated (∼30-fold) serum-free conditional culture medium of the cells transfected with pcDNA3-ATX-Myc and the lysate of the cells transfected with pcDNA3-ATXFF/AA-Myc were treated with Endo H and then analyzed by Western blotting with anti-Myc antibody. Data are representative of three independent experiments.