Figure 11.
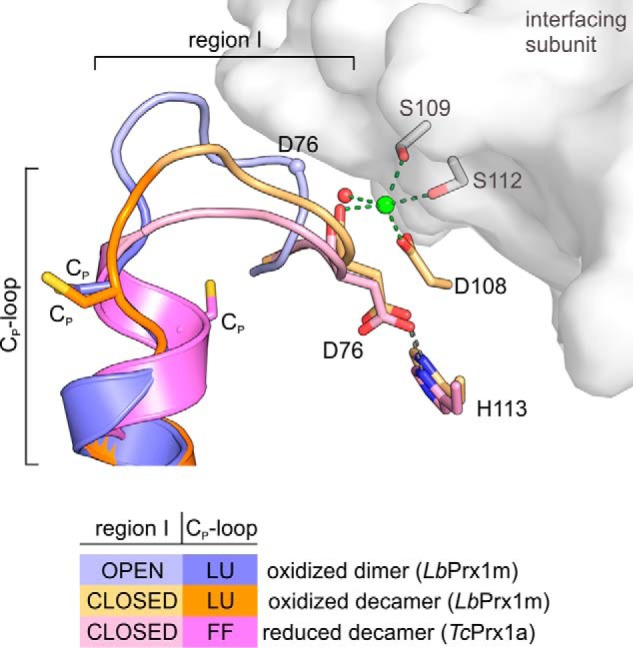
Stabilization of region I in the closed conformation is required to maintain the fully folded conformation of the Cp-loop upon Cp reduction. Shown is a 3D alignment of the loop-helix active-site motif (region I + Cp-loop) of LbPrx1m-oxidized dimer (shades of violet; PDB accession no. 4KCE), LbPrx1m-oxidized decamer (shades of orange; PDB accession no. 4KB3) after molecular dynamics simulations with Ca2+ bound (green sphere) at the A-type interface (gray surface), and reduced decamer TcPrx1a (shades of pink; PDB accession no. 4LLR). Note that region I adopts an open conformation in dimers, but it assumes a closed conformation in the oxidized and reduced decamers. According to our data, the stabilization of region I in the closed conformation requires at least an H-bond between Asp-76 and His-113 as well as Ca2+ (green sphere), Mg2+, or (less efficiently) the water molecule at the A-type interface. The establishment of such interactions is crucial to maintain the fully folded (FF) form of the reduced Cp-loop. In the LbPrx1m dimer, the high entropy of the region I, trapped in an open conformation in chain B but disordered in chain A, might propagate to the adjacent Cp-loop, hampering the stabilization of fully folded conformation required for substrate binding and catalysis. LU, locally unfolded.
