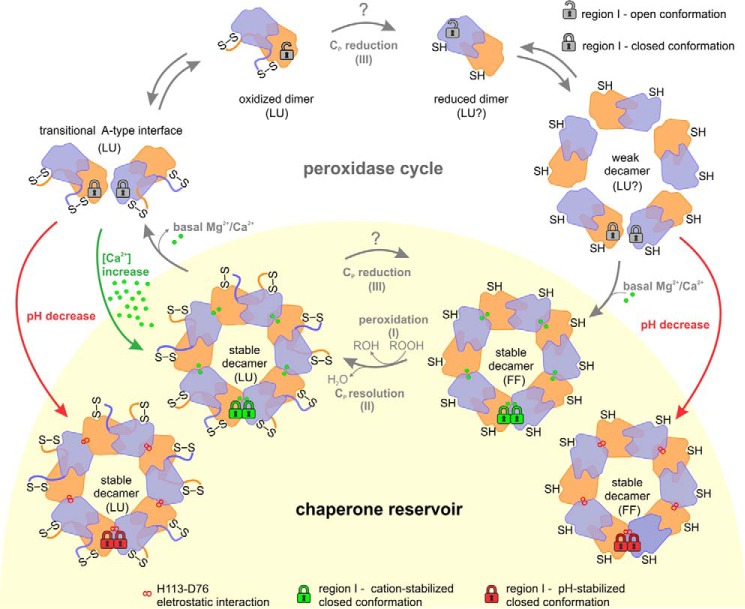
Figure 13.
Physiological role of Mg2+/Ca2+, pH, and redox state in the maintenance of the chaperone reservoir of mitochondrial Prx1 in Leishmania. During the peroxidase cycle, Leishmania Prx1m transits between the dimeric (chaperone-inactive) and the decameric (chaperone-active) forms. This oligomeric shift is regulated by the conformational changes of two regions from the loop-helix active-site motif: the Cp-loop (LU ⇔ FF), which is redox-sensitive, and the region I (open ⇔ closed), which is Ca2+/Mg2+/pH-sensitive. When dimers are reduced (SH), they tend to form weak decamers in which free Mg2+ and Ca2+ ions bind to enhance their peroxidase and chaperone functions. In basal conditions, after the peroxidation (I) and resolution (II) steps, oxidized (S–S) decamers tend to release Mg2+ and Ca2+ ions and dissociate into dimers, a process that involves an intermediate state, here named the transitional A-type interface. However, under heat-stress conditions, two stimuli can boost the chaperone reservoir of Prx1m, mainly by stabilizing the oxidized decamers: red arrows, small pH decreases; green arrows, Ca2+ overload. Because our data could not discriminate the preferable substrate for tryparedoxin (Prx1m dimers or decamers), we labeled the Cp reduction step with a question mark. The same is applicable for the conformational state proposed for the active site in reduced dimers and reduced weak decamers. For purposes of clarity, the padlocks illustrating the conformational state of region I are shown only for a pair of dimers at the decamers. LU, locally unfolded; FF, fully folded.