Figure 4.
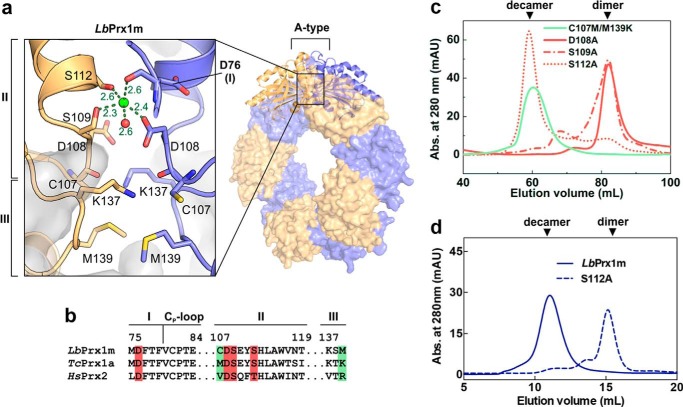
Structural basis for the Ca2+/Mg2+-dependent decamerization of LbPrx1m. a, magnified view of the A-type interface from LbPrx1m crystal structure (PDB accession no. 4KB3) after molecular dynamics simulations in the presence of Ca2+ (green sphere). Interfacing subunits are shown in orange and violet. For purposes of clarity, we named the A-type interface regions according to the nomenclature proposed by Wood et al. (15) (regions I–III). Shown as sticks are the residues involved in the cation-dependent mechanism of decamer stabilization: Asp-76, region I; Cys-107, Asp-108, Ser-109, and Ser-112, region II; Lys-137 and Met-139, region III. b, sequence alignment of A-type interface regions from LbPrx1m and two cation-independent 2-Cys Prx, highlighting the residues predicted to play a role in cation binding (red boxes) and those predicted to determine the cation dependence of decamer stabilization (green boxes). c, aSEC chromatograms of air-oxidized LbPrx1m D108A, S109A, S112A, and C107M/M139K mutants at 86 μm (500 μl) in Tris buffer (pH 7.5) containing 25 mm CaCl2 (red lines) or 5 mm EDTA (green line). The single mutant C107M was unstable in solution, and thus it was not included in our analyses. mAU, milliabsorbance units. d, aSEC chromatogram of air-oxidized LbPrx1m WT and S112A mutant at 13 μm (200 μl). Note that WT protein eluted as decamer, whereas the S112A mutant eluted mainly as dimer under the same conditions. aSEC assays were carried out in Tris buffer (pH 7.5) containing 25 mm CaCl2.