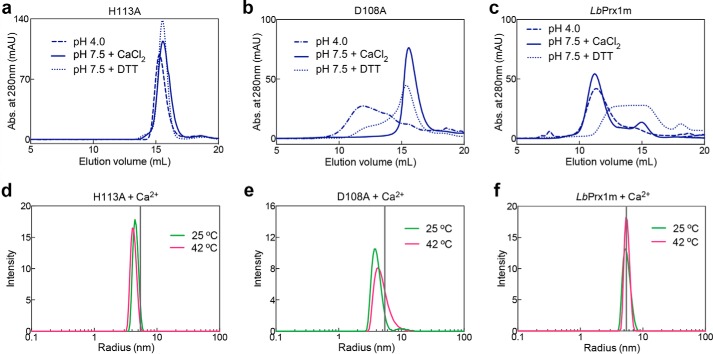
Figure 7.
The influence of low pH, CaCl2, DTT, or temperature on the hydrodynamic behavior of the mutants H113A and D108A and WT LbPrx1m. aSEC assays of H113A (a), D108A (b), and WT LbPrx1m (c) were carried out with proteins at 48 μm (250 μl) in MMT buffer (pH 4.0) and 10 mm EDTA (dashed-dotted line), Tris buffer (pH 7.5) and 25 mm CaCl2 (solid line), or Tris buffer (pH 7.5) and 5 mm EDTA containing 2 mm DTT (dotted line). Chromatograms of mutant H113A and LbPrx1m at pH 4.0 as well as LbPrx1m at pH 7.5 plus 2 mm DTT were shown previously (9) and are represented here for comparison purposes. DLS analyses at 25 and 42 °C of H113A (d), D108A (e), and WT LbPrx1m (f) were performed with protein samples at 100 μm pretreated with 5 mm EDTA followed by the addition of 25 mm CaCl2 in Tris buffer (pH 7.5). The vertical gray lines represent the mean radius estimated for WT LbPrx1m decamers. Samples not treated with DTT are air-oxidized (S–S-bonded). mAU, milliabsorbance units.