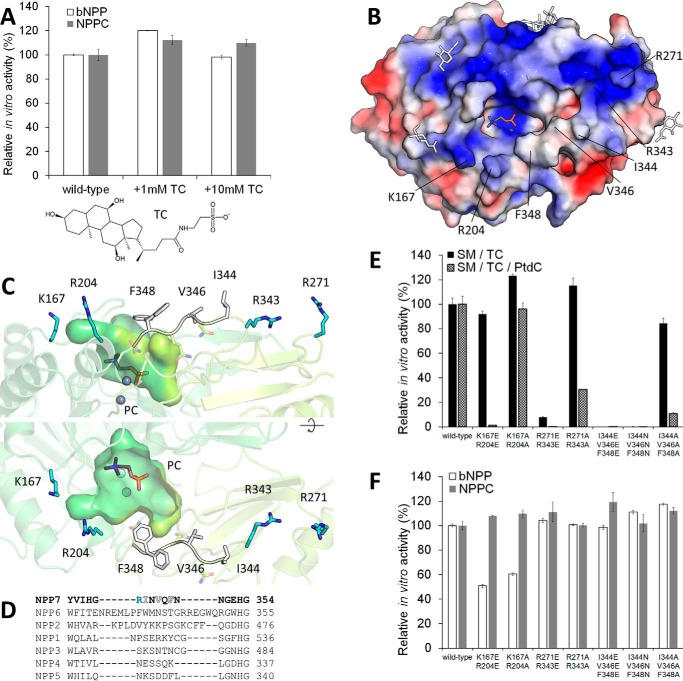
Figure 3.
Regions of alk-SMase important for bile salt-dependent activity. A, enzymatic activity of purified alk-SMase on substrates in presence of TC (chemical structure displayed). 100% activity corresponds to 2.49 and 50.26 μm of p-nitrophenol released per nm protein per hour from bNPP and NPPC, respectively. B, the electrostatic surface potential of alk-SMase is colored from negative (−3 kT/e, red) to positive (+3 kT/e, blue). PC is shown as gray sticks and N-linked glycans, as simplified white sticks. C, residues near the substrate binding site that form cationic patches (blue sticks) and hydrophobic residues of the loop 342–351 (white sticks) are labeled. Two conformations of Phe-348 are visible. D, loop 344–348 of alk-SMase (NPP7) is aligned with corresponding regions of the other NPPs. E and F, enzymatic activity of purified alk-SMase on substrates. 100% activity corresponds to 2.58 and 61.68 μm of p-nitrophenol and 44.72 and 41.80 μm of PC released per nm protein per hour from bNPP, NPPC, and SM in TC micelles and SM in mixed TC-phosphatidylcholine (PtdC) micelles, respectively. For A, E, and F, the values are the means and standard deviations of triplicates representative of one of two experiments.