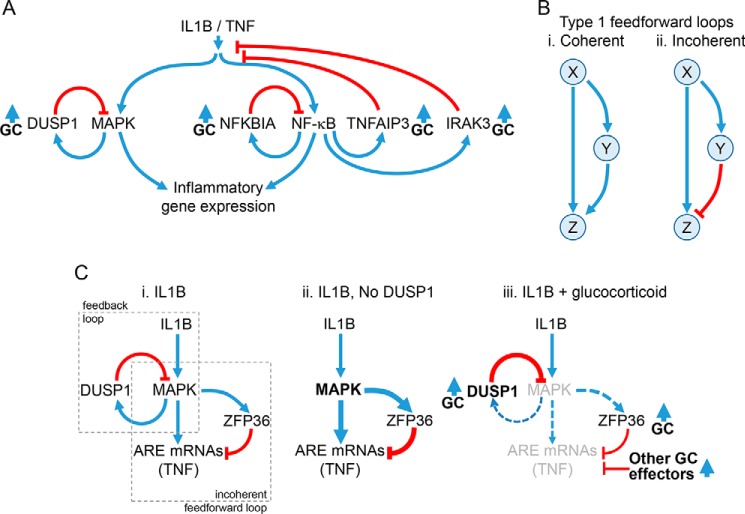
Figure 2.
Regulatory loops controlling inflammatory gene expression. A, schematic showing activation of MAPK pathways and NF-κB by IL1B or TNF leading to the expression of inflammatory genes. MAPKs induce the expression of the phosphatase, DUSP1, which provides feedback control to switch off MAPK activity. NF-κB binds κB sites in promoters of target genes. This activates transcription of NFKBIA, TNFAIP3, and IRAK3 to increase their expression and leads to feedback inhibition of NF-κB or IL1B/TNF signaling. Expression of DUSP1, NFKBIA, TNFAIP3, and IRAK3 can also be enhanced by glucocorticoids (GC). B, type I coherent and incoherent feedforward loops are depicted. In the type I coherent feedforward loop (panel i), X positively regulates Y, and Z is positively regulated by both X and Y. In the type I incoherent feedforward loop (panel ii), X positively regulates both Y and Z, but Y negatively regulates Z. C, schematic showing how feedback and feedforward regulation may interplay to regulate AU-rich element (ARE)-containing inflammatory mRNAs. Panel i, pro-inflammatory stimuli, here IL1B, activate MAPK pathways, leading to the expression of ARE-containing mRNAs, such as TNF. MAPK activation not only also induces expression of the feedback regulator, DUSP1, but also promotes expression of ZFP36. ZFP36 is a feedforward regulator that leads to mRNA destabilization of ARE-containing mRNAs, such as TNF. Thus, the MAPK-dependent induction of ZFP36 leads to repression of ARE-containing mRNAs and constitutes a classic type I incoherent feedforward loop. Note that expression of ARE-containing mRNAs and ZFP36 is also likely to involve NF-κB, and this is not depicted. Panel ii, Following loss, or silencing, of DUSP1, MAPK activity is enhanced and leads to increased expression of downstream genes. However, expression of ZFP36 is also enhanced, and this acts to reduce expression of ARE-containing mRNAs, such as TNF. Panel iii, ZFP36 expression is up-regulated by glucocorticoids alone, but ZFP36 expression induced by the inflammatory stimulus is reduced by glucocorticoid, in part due to reduced MAPK activity following the induction of DUSP1. Although these effects may combine to promote expression of the hypo-phosphorylated and more active, mRNA-destabilizing form of ZFP36, silencing of both DUSP1 and ZFP36 showed little effect on the repression of TNF by glucocorticoid. Additional, glucocorticoid-induced effector processes are therefore likely to play additional repressive roles.