Figure 1.
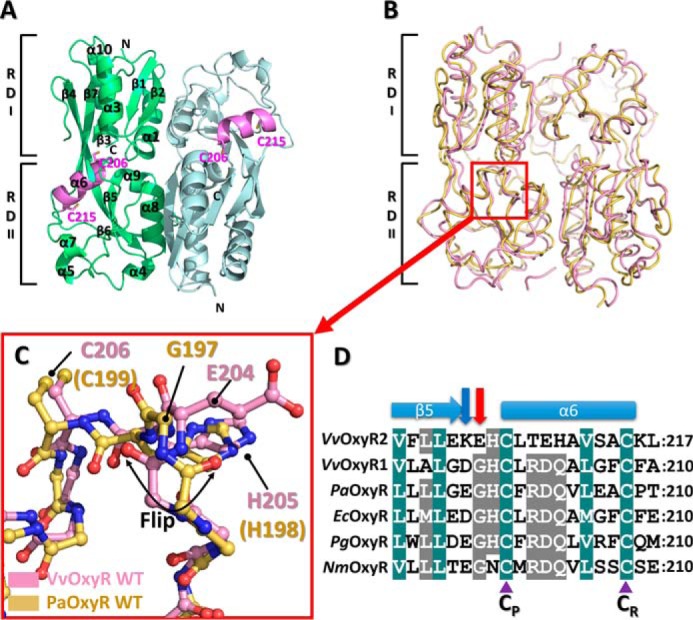
Structure of the wild-type VvOxyR2-RD. A, dimeric assembly of wild-type VvOxyR2-RD under reduced conditions. Protomers are highlighted in green or light blue, except in the inter-cysteine α-helical regions (pink). The conserved cysteines (Cys-206 and Cys-215) are represented as sticks. Secondary structural elements and the subdomains RD-I and RD-II are labeled. B, comparison of VvOxyR2-RD dimers (pink) with P. aeruginosa OxyR-RD in the reduced state (yellow; PDB code 4Y0M): The red box indicates the region near the peroxidatic cysteine (Cys-206) and the Glu-204-containing loop. C, close-up view of the region indicated by the red box in B. The regions near the peroxidatic cysteine of VvOxyR2-RD (pink) and PaOxyR-RD (yellow) are shown in a ball-and-stick representation. The flipped peptide bond is indicated with a double-headed arrow. D, sequence alignment of VvOxyR2 with other OxyRs, focusing on the region containing the conserved cysteine residues (V. vulnificus (Vv), P. aeruginosa (Pa), and N. meningitidis (Nm)). The secondary structures are displayed above the sequence. Glu-204 or its equivalent residue and Lys-203 or its equivalent residue are indicated by a red or a blue arrow, respectively. Two conserved cysteine residues (peroxidatic cysteine (CP) and resolving cysteine (CR)) are indicated by purple triangles. The strictly conserved amino acid residues are indicated by cobalt blue boxes, and the moderately conserved amino acid residues are indicated by gray boxes.
