Figure 3.
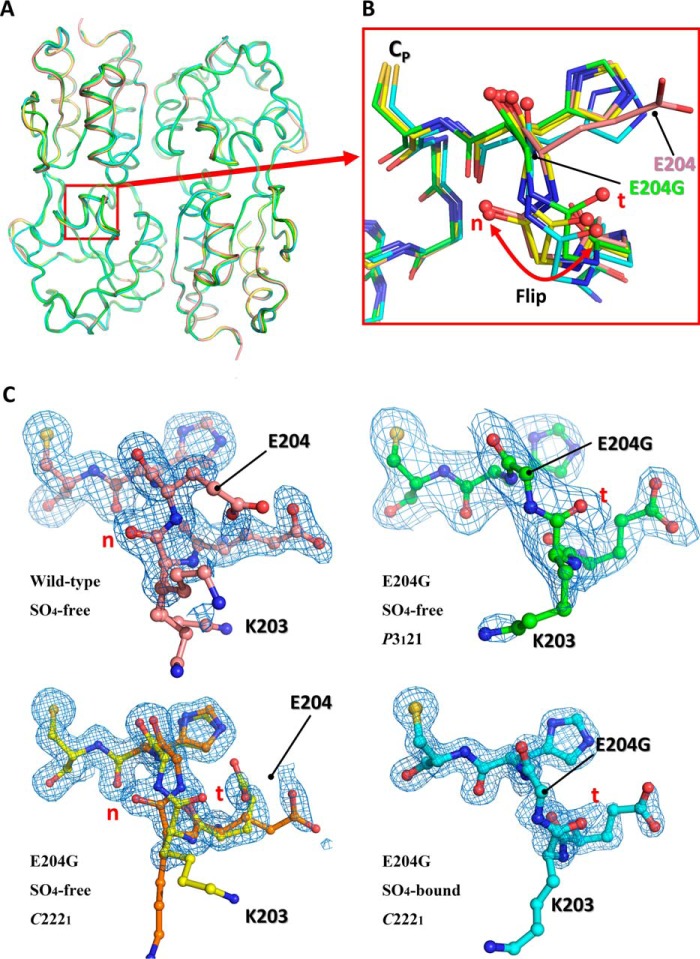
Structural comparison of the wild-type VvOxyR-RD and the VvOxyR2-RD (E204G) variant. A, structural superposition of the wild-type VvOxyR2-RD (pink) and the three VvOxyR2-RD (E204G) variants that are named SO4-free P3121 (green), SO4-free high-resolution (yellow and orange), and SO4-bound (cyan). Regions containing the peroxidatic cysteine and the Glu-204-containing loop are indicated by a red box. B, a close-up view of the red boxed region in A; all residues are drawn as ball-and-stick representations. A flipped peptide bond is indicated with a double-headed arrow. t and n letters in red indicate the typical conformation observed in the most OxyR proteins and the noncanonical conformation only observed in the wild-type VvOxyR2, respectively. C, 2Fo − Fc electron density maps (blue mesh) around the Glu-204-containing of the wild-type (top left, pink), SO4-free high-resolution E204G (bottom left, yellow and orange), SO4-free P3121 E204G (top right, green), and SO4-bound E204G variant (bottom right, cyan) are contoured at 1.0 σ. t and n letters in red indicate the typical conformation observed in the most OxyR proteins and the noncanonical conformation only observed in the wild-type VvOxyR2, respectively.