Coronary heart disease (CHD) and myocardial infarction (MI) account for approximately 1 in 7 deaths in the US, with an estimated 750,000 incidences of MI in the US each year1. Although modern medical therapies have reduced the number of CHD-related deaths over the past thirty years, there is still much room for improvement in CHD and MI treatment. As cardiac tissue has limited regenerative capability, damaged myocardial tissue downstream of the blocked vessel in MI remodels to nonfunctional fibrotic scar tissue. Cell and tissue-engineered therapies are a promising therapeutic area that may reduce MI scar formation and induce healthy remodeling of damaged heart tissue by providing cells and matrix materials that can integrate with native tissue to restore normal heart function.
Tissue engineering takes advantage of a wide range of technologies, including stem cell techniques and 3D printing, to recapitulate in vivo tissue structure and function in an in vitro setting. The primary focus of tissue engineering research is regenerative medicine applications that aim to implant cells in a biomaterial scaffold into a diseased or injured tissue and thereby restore tissue functionality. To accomplish this, the tissue-engineered construct must be designed to ensure cell survival by either replacing damaged native tissue or being resorbed as new tissue forms, without inducing immunogenicity.
Myocardial tissue engineering is particularly challenging due to the complex nature of the heart’s structure and function. Implanted myocardial tissue constructs should ideally integrate into the surrounding heart tissue both physically and functionally, with implanted CMs coupling with neighboring native cells in contraction and electrical signal conduction without inducing arrhythmias. However, to functionally couple with native tissue, the cells in the implanted tissue construct must first survive. This has been one of the biggest roadblocks to successful cell and tissue engineering therapies for cardiac diseases, especially in the case of cells injected without a scaffold, a large portion of which do not survive in the days to weeks after treatment2,3. While these low levels of cell survival may still result in effective treatment and improved heart muscle function after ischemic injury3,4, improved cell survival should in principle significantly improve recovery or at least reduce the cell number required for transplantation. In an effort to improve cell survival, Gao and colleagues describe in this issue of Circulation Research the generation of a native tissue-like cardiac muscle patch scaffold using multiphoton-excited, 3-dimensional printing (MPE-3DP)5.
Cardiac muscle patches (CMPs), alternatively referred to as engineered heart tissues (EHTs) or engineered cardiac tissues (ECTs), have a wide range of promising applications in disease modeling, drug testing, and regenerative therapies. The primary components of CMPs include an extracellular matrix (ECM)-based scaffold, typically composed of fibrin or collagen, and cardiomyocytes (CMs). Additional cell types, such as endothelial cells, smooth muscle cells, and fibroblasts, are often included to make the CMP cell composition more representative of the natural cell composition of the heart (Figure 1). Early CMP protocols produced an ECM scaffold by mixing cells with protein solutions, primarily collagen I, and allowing the solution to gel with the cells inside6,7. Because this unstructured gel is not representative of the natural structure of the heart ECM, the investigators took advantage of MPE-3DP technology to attempt to recapitulate the native extracellular matrix (ECM) structure at a submicron resolution.
Figure 1. Generation of cardiac muscle patches.
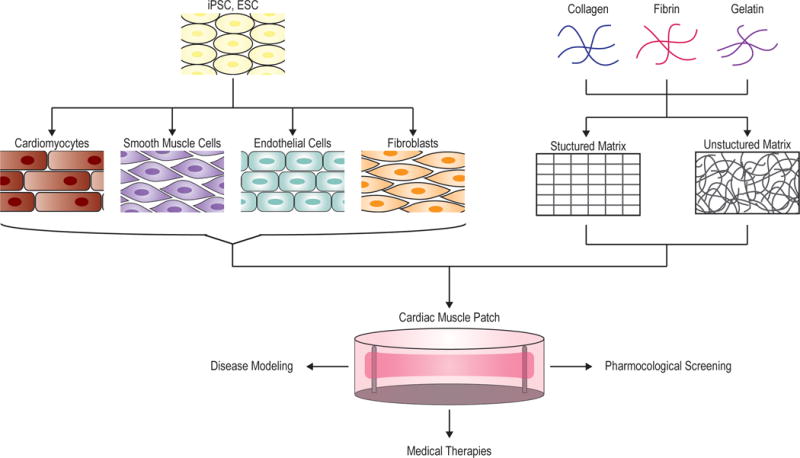
CMPs are generated from a combination of cells and extracellular matrix material. iPSCs or ESCs are differentiated into the cell types commonly found in the heart, which include cardiomyocytes, endothelial cells, smooth muscle cells, and fibroblasts. These iPSC- or ESC-derived cells are then mixed at a ratio representative of their composition in native heart tissue. The cells can be directly seeded into a prefabricated, structured scaffold or mixed with a solution of extra cellular matrix proteins that will gel to form an unstructured matrix with the cells inside. Common matrix materials include fibronectin, collagen, and gelatin. The CMP can then be kept in culture for disease modeling and pharmacological screens, or can be transplanted onto a diseased heart as a regenerative therapy.
In general, 3D printing is being increasingly used in tissue engineering both to design acellular scaffolds and to directly print mixtures of biomaterials and live cells. Previous 3D printing technology could produce of scaffolds with approximately 20 μm resolution, allowing creation of large tissue engineered constructs, such as bone and trachea, with accurate microarchitecture8. MPE-3DP uses a laser to excite and crosslink photoactive biopolymers or proteins, with control over crosslinking in all three dimensions that allows for extremely fine, submicron resolution of the final scaffold. This technique has been used to print scaffolds of biomaterials such as fibronectin, which has been shown to allow cell adhesion9. For the base of their scaffold, Gao and colleagues used gelatin methacrylate, which is a photoactivatable gelatin-based polymer that contains natural cell-binding and degradation sites10. The authors designed a scaffold template as a grid-based native adult murine ECM structure, in which fibronectin is uniformly distributed around the cells. This template, in the form of an image, was mapped by modulated raster scanning as crosslinks in the gelatin methacrylate solution based on the intensity of each peak of the image, forming a reproducible, robust scaffold.
In their MPE-3DP scaffold, the investigators used human induced pluripotent stem cell (hiPSC)-derived CMs, endothelial cells, and smooth muscle cells to create a human CMP. CMs, the primary differentiated cell type in heart muscle, are quiescent, making them difficult to maintain as primary cells in long-term in vitro culture for tissue engineering applications. Instead, hiPSCs in combination with embryonic stem cells (ESCs) have been used to generate CMs for tissue-engineered myocardium. One of the biggest barriers to using hiPSC- or ESC-derived CMs for disease modeling and medical therapies is their immaturity. Relative to adult cardiomyocytes, hiPSC- and ESC-CMs are smaller and more rounded, exert significantly smaller contractile forces, express a fetal-like transcriptome, and exhibit differences in calcium handling and mitochondrial structure11. While it does not completely mature the CMs to the adult phenotype, culturing hiPSC-CMs or ESC-CMs in tissue engineered constructs such as CMPs improves CM contractile force and sarcomere alignment and results in more adult-like gene expression12. Gao et al. illustrated that hiPSC-CMs seeded in their scaffold exhibited functional maturation after 7 days, with increased levels of calcium handling and contractility gene expression compared to monolayer culture, along with multinucleation, alignment, and elongation of cells within the channels of the scaffold to a morphology similar to that observed in native cardiac tissue. The authors also showed that the CMP constructs generated calcium transients and exhibited synchronous beating within one day after seeding, with improvements in both characteristics over the following 7 days of culture.
To test the efficacy of MPE-3DP CMPs as a regenerative therapy for MI, Gao and colleagues transplanted CMPs onto the site of surgically induced MI in mice. Animals either received MI with two CMPs on the site of myocardial injury, MI with two CMP scaffolds without cells, MI with no treatment, or a sham surgery with no induced MI. In the group which received CMPs, engraftment of transplanted cells averaged 24.5% after one week and decreased by week 4 to 11.2% as measured by PCR or 13.6% as measured by histological assessment. There was also a significant decrease in the ratio of CMs to endothelial cells and smooth muscle cells over the course of the four weeks, likely due to the limited proliferative ability of quiescent CMs compared to the other cell types. Even with the limited cell engraftment, Gao et al. found that MI mice treated with CMPs showed a significantly improved ejection fraction and fractional shortening at four weeks compared to the MI and MI plus scaffold groups. The authors also observed that the infarct area was smaller with a significantly thicker myocardial wall, and found evidence of decreased apoptosis and increased angiogenesis and proliferation.
The next step for Gao and colleagues would be to evaluate the electromechanical coupling of their CMP to native heart tissue. The fact that the CMPs exhibited spontaneous contraction and calcium transients just one day after cell seeding and continuous action potential propagation across the CMPs at 7 days suggests that the cells would likely be able to transduce the electromechanical signaling of neighboring tissues. Another potential area to focus future studies is further improvement in cell survival and clinical outcomes, which could include exploration of alternative methods for functional maturation of the CMPs prior to implantation and investigation of soluble factors or matrix components that individually improve post-MI remodeling of native tissue. Functional maturation is a particularly relevant issue not only for medical treatment, but also for disease modeling and drug screening, for which physiologically relevant responses of tissue engineered constructs are required. Promising methods for maturation include extended culture times, electrical pacing, and inducing mechanical strain13,14.
In addition to the needed improvements in cell survival and treatment efficacy in clinical trials, another significant barrier to large-scale clinical applicability of cell and tissue engineered therapies for the heart is the scalability and storage issues associated with live cell culture. If cell therapies, especially those which use terminally differentiated cells such as CMs, are to be used in clinical treatment, methods for large batch culture and quality control for increased purity and reduced batch-to-batch variations in cell phenotype must be established15. Nevertheless, on a smaller scale, CMPs can be used for other applications, such as drug screening and disease modeling, in which the resemblance between engineered constructs and native tissue is important for recapitulating physiological responses. While there is still a long way to go for attaining accessible clinical use of this technology, the innovative use of MPE-3DP technology represents a significant advancement in myocardial tissue engineering.
Acknowledgments
This publication was supported in part by research grants from the National Institutes of Health NIH R01 HL133272, NIH R01 HL132875, and NIH R01 HL128170 (J.C.W.).
Footnotes
Disclosures
None
References
- 1.Mozaffarian D, et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation. 2015;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: From bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riegler J, et al. Human engineered heart muscles engraft and survive long-term in a rodent myocardial infarction model. Circ Res. 2015;117:720–730. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye L, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–61. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao L, et al. Myocardial tissue engineering with cells derived from human induced-pluripotent stem cells and a native-like, high-tesolution, 3-dimensionally printed scaffold. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.116.310277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eschenhagen T, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 7.Tzatzalos E, Abilez OJ, Shukla P, Wu JC. Engineered heart tissues and induced pluripotent stem cells : Macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliv Rev. 2016;96:234–244. doi: 10.1016/j.addr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E. A review of three-dimensional printing in tissue engineering. Tissue Eng Part B Rev. 2016;22:298–310. doi: 10.1089/ten.TEB.2015.0464. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Su YD, Ajeti V, Chen SJ, Campagnola PJ. Cell adhesion on micro-structured fibronectin gradients fabricated by multiphoton excited photochemistry. Cell Mol Bioeng. 2012;5:307–319. doi: 10.1007/s12195-012-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichol JW, Koshy S, Bae H, Hwang CM, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sayed N, Liu C, Wu JC. Translation of human-induced pluripotent stem cells: From clinical trial in a dish to precision medicine. J Am Coll Cardiol. 2016;67:2161–2176. doi: 10.1016/j.jacc.2016.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, et al. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34:5813–5820. doi: 10.1016/j.biomaterials.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirt MN, et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol. 2014;74:151–161. doi: 10.1016/j.yjmcc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Salazar BH, Cashion AT, Dennis RG, Birla RK. Development of a cyclic strain bioreactor for mechanical enhancement and assessment of bioengineered myocardial constructs. Cardiovasc Eng Technol. 2015;4:533–545. doi: 10.1007/s13239-015-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempf H, Andree B, Zweigerdt R. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv Drug Deliv Rev. 2016;96:18–30. doi: 10.1016/j.addr.2015.11.016. [DOI] [PubMed] [Google Scholar]


