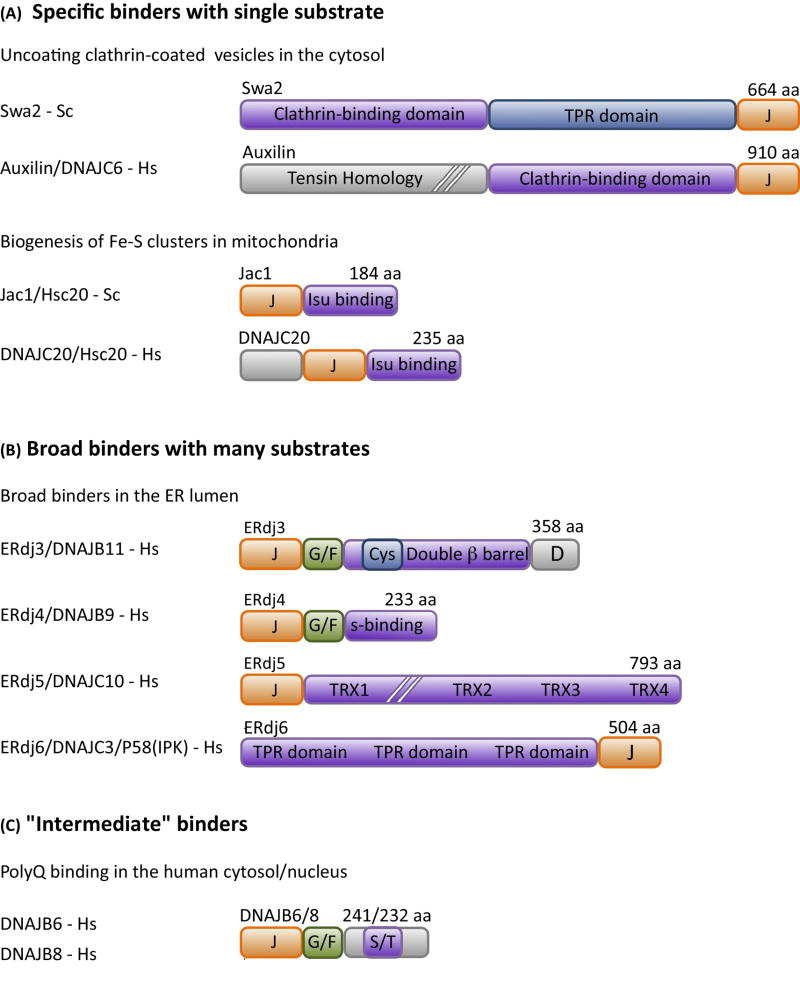
Figure 3. Diverse modes of binding of substrate by J-proteins.
J-proteins have structurally diverse substrate binding domains, which range in binding specificity from (A) very selective (i.e. in some cases a single substrate), that is “specific binders” to (B) quite promiscuous, binding to most any short hydrophobic stretch of amino acids, that is “broad binders”. The 4 “broad binders” of the human ER lumen are shown. ERdj3 is a double β-barrel protein (see Box 3). ERdj4 has an ill-defined substrate binding domain (S-binding). Erdj5 and ERdj6 have thioredoxin repeats (TRX) with reductase activity and tetratricopeptide repeats (TPR), respectively, but exact sites of substrate binding are yet to be well defined. (C) Many J-proteins do not fall neatly into these two categories. The DNAJB6/8 J-proteins that bind polyglutamine (polyQ) stretches are an example of the less well-defined intermediate class; the serine/threonine-rich (S/T) region is the experimentally defined region critical for polyQ binding, but it is dispensable for binding of another substrate, as described in the text. As the J-protein family as a whole becomes better characterized, well defined classes of J-proteins based on binding specificity will likely be difficult to delineate, as the binding specificity spectrum is likely a continuous one. Most common names of J-proteins S. cerevisiae (Sc) and H. sapiens (Hs) used in the literature are listed. Domain organization of representative J-proteins is drawn to scale, except where indicated by hatch (//) marks; J- J-domain; G/F- glycine/phenylalanine rich region; Cys-cysteine-rich region.