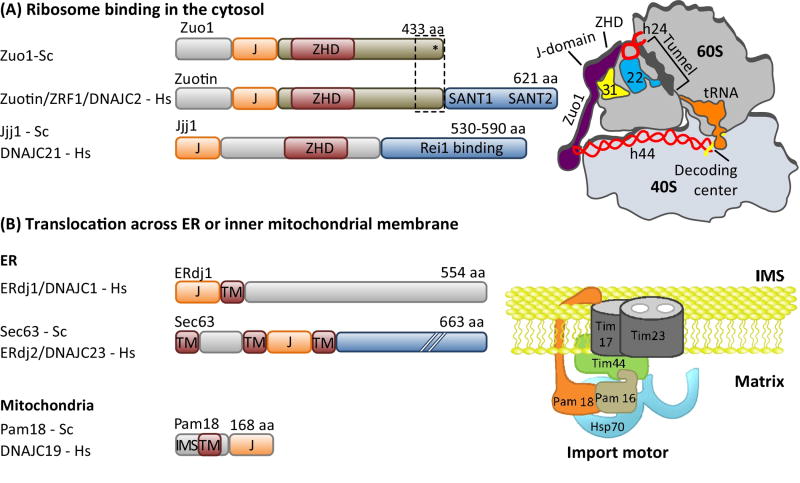
Figure 4. Localization of J-proteins to specific sites within a compartment.
J-proteins that localize (A) near the opening ribosome exit tunnel and (B) at the import channel (translocon) in the ER and inner mitochondrial membranes do not bind substrates, but help recruit and orient their partner Hsp70 for effective substrate binding. (A) One ribosome-associated J-protein, called Zuo1/Zuotin, interacts with both subunits via indicated protein (eL31, yellow) and rRNAs (red) (right). Interaction with the 60S subunit is via the Zuotin homology domain (ZHD). Regions of Zuo1/Zuotin towards the C-terminus (boxed) are important for interaction with the 30S subunit; ribosomal protein ul22 (22) interacts both with helix 44 (h24) and the interior of the tunnel, serving as a potential site for monitoring the nascent chain. As discussed in the text, the architecture and function of Zuo1/Zuotin illustrate how some J-proteins have evolved complex activities; C-terminal SANT domains of human and the 13 C-terminal residues of Zuo1 (*) are both involved in cellular regulation. A second J-protein (Jjj1/DNAJC21) also interacts with eukaryotic 60S ribosome particles via its ZHD (bottom). Jjj1 serves as an example of the difficulties in rigorously categorizing J-proteins by their structure and/or client binding properties. While localized to its site of action, it also binds a specific substrate protein, Rei1, as discussed in the text. (B) J-protein functioning in protein translocation are integral membrane proteins and also interaction with other proteins of the translocation apparatus. In the case of the import motor of the mitochondrial inner membrane (right), Pam18/DNAJC19 interacts with translocon subunit Tim17 on the intermembrane space (IMS) side and indirectly on the matrix side (with the import motor component Pam16 directly; Pam16 directly interacts with Tim44, which directly interacts with the import channel). J-protein names from S. cerevisiae (Sc) and H. sapiens (Hs) are listed. Domain organization of J-proteins is drawn to scale except where indicated by hatch marks (//); J- J-domain; TM- transmembrane region.