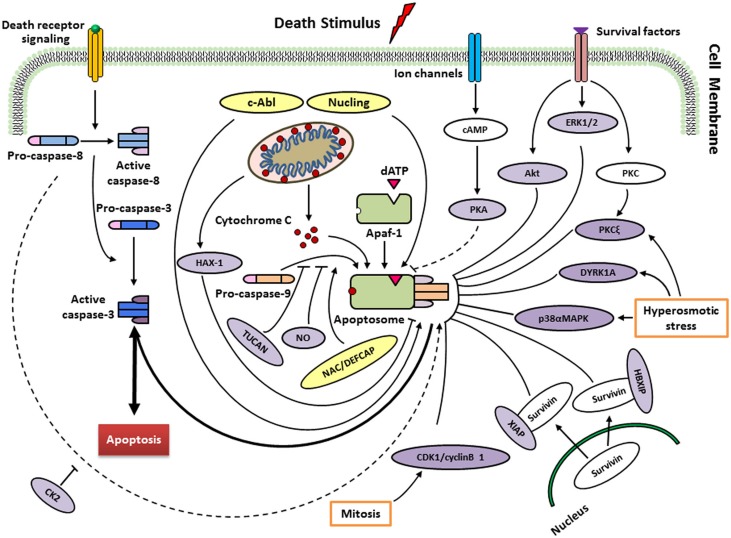
Figure 2. Regulation of caspase-9 by endogenous regulators in different signalling pathways.
Following cytochrome c release from mitochondria, a heptameric wheel-like multimeric complex, the apoptosome, induced and composed by Apaf-1 and procaspase-9. Factors such as ATP, can inhibit this process by directly inhibiting the interaction between Apaf-1 and cytochrome c. Recruitment of procaspase-9 to the apoptosome is antagonized by TUNCAN, and stimulated by NAC/DEFCAP. HAX-1 may inhibit caspase-9 activation, thereby suppressing apoptosis in cardiac myocytes. Direct phosphorylation at Thr125 by ERK1/2 (growth/survival signals), CDK1-cyclin B1 (in mitosis), p38αMAPK (upon hyperosmotic stress) and DYRK1A (in development and upon hyperosmotic stress) inhibits caspase-9 activity. PKCξ also inhibits phosphorylation of caspase-9 at Ser144 induced by hyperosmotic stress. Akt/PKB, as a protein kinase, suppresses the activation of caspase-9 in response to extracellular growth/survival signals. The interactions of HBIXP and XIAP with survivin block the activation of caspase-9 through distinct mechanisms. PKA seems to block the recruitment of caspase-9 to apoptosome rather than inhibitory effect on caspase-9 activation (unclear). CK2 phosphorylates caspase-9 at Ser348 to protect from caspase-8 cleavage in mouse, but not conserved in humans. Nitrosylation of caspase-9 by the donor of NO suggests cleavage inhibition of procaspase-9 and consequently apoptosis. Conversely, phosphorylation at Tyr153 by c-Abl stimulates activiation of caspase-9. Nucling recruits and transports the apoptosome when responding to the apoptosis induced by stresses. See text for more details.