Figure 3.
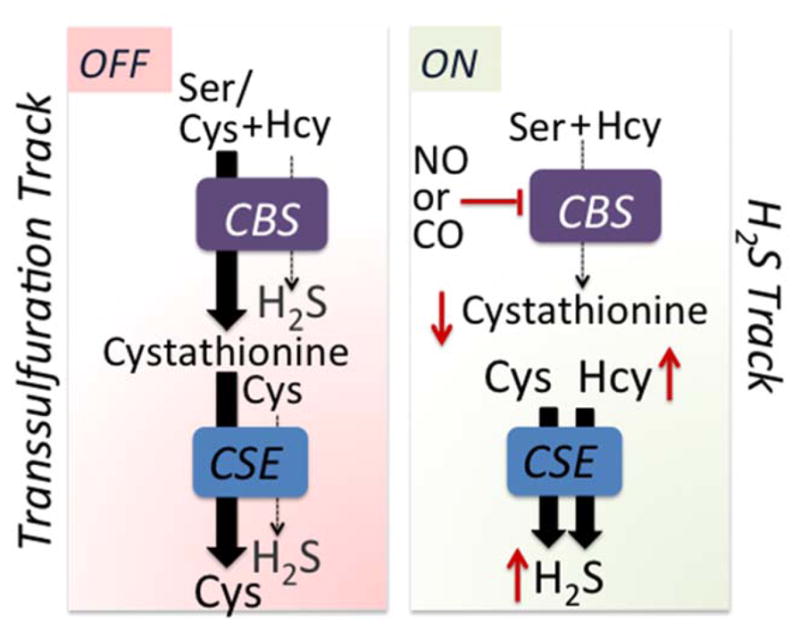
Heme-dependent metabolic track switching. The canonical transsulfuration track operates when the heme in CBS is coordinated by its endogenous ligands and serine, which is more abundant than cysteine and binds with higher affinity, competes effectively for the active site. The product, cystathionine, is then converted by CSE to cysteine. The enzymes switch metabolic tracks when ferrous CBS binds either NO or CO, inhibiting activity, which leads to an increase in homocysteine and a decrease in cystathionine. Under these conditions, H2S synthesis from cysteine, which is catalyzed by CSE, is promoted. The red up and down arrows denote changes in metabolite levels.
