Abstract
Background
There is limited evidence regarding the optimal timing of initiating antiretroviral therapy (ART) in children. We conducted a causal modelling analysis in children aged 1–5 years from the International Epidemiologic Databases to Evaluate AIDS West/Southern-Africa collaboration to determine growth and mortality differences related to different CD4-based treatment initiation criteria, age groups and regions.
Methods
ART-naïve children of age 12–59 months at enrollment with at least one visit before ART initiation and one follow-up visit were included. We estimated 3-year growth and cumulative mortality from the start of follow-up for different CD4 criteria using g-computation.
Results
About one quarter of the 5826 included children was from West Africa (24.6%). The median (first; third quartile) CD4% at the first visit was 16% (11%;23%), the median weight-for-age z-scores and height-for-age z-scores were −1.5 (−2.7; −0.6) and −2.5 (−3.5; −1.5), respectively. Estimated cumulative mortality was higher overall, and growth was slower, when initiating ART at lower CD4 thresholds. After 3 years of follow-up, the estimated mortality difference between starting ART routinely irrespective of CD4 count and starting ART if either CD4 count<750 cells/mm3 or CD4%<25% was 0.2% (95%CI: −0.2%;0.3%), and the difference in the mean height-for-age z-scores of those who survived was −0.02 (95%CI: −0.04;0.01). Younger children aged 1–2 and children in West Africa had worse outcomes.
Conclusions
Our results demonstrate that earlier treatment initiation yields overall better growth and mortality outcomes, though we could not show any differences in outcomes between immediate ART and delaying until CD4 count/% falls below750/25%.
INTRODUCTION
Despite a reduced number of newly infected children in 2012, the burden of HIV remains high with 260,000 new annual pediatric infections in low- and middle-income countries.1 The optimal timing of antiretroviral treatment (ART) initiation in children beyond 12 months of age remains controversial: early ART initiation may reduce morbidity and mortality but could increase the risk of toxicity, complications due to non-adherence, and early development of drug resistance.2–6
The World Health Organization (WHO) 2006 guidelines recommended treatment initiation for all children with WHO clinical stage III/IV (with exceptions for children ≥12months, stage III, and particular clinical events) or based on age-dependent CD4 criteria for children with clinical stage I/II (starting ART if (i) CD4 count<350 cells/mm3 or CD4%<15% for children aged 36–59 months (ii) CD4 count<750 cells/mm3 or CD4%<20% for children aged 12–35 months). The CHER trial showed a 76% (95% CI: 49%–89%) reduction in mortality in infants, enrolled at age 6–12 weeks, for immediate ART initiation versus deferring ART until CD4% was lower than 25%.7 These results caused WHO to update their guidelines in 2008 to recommend ART initiation in all HIV-infected children less than 12 months of age, regardless of their clinical and immunological status. These recommendations were expanded to all HIV-infected children less than 24 months of age in 2010 while for children between 24–59 months, with an asymptomatic or mild clinical disease, ART was recommended if either CD4 count<750 cells/mm3 or CD4%<25%. Both the 2006 and the 2010 recommendations relied however solely on the evaluation of disease progression in analyses that were neither randomized experiments nor causally interpretable. In addition, many of these analyses were based on data from high-income countries.8–11
The question of when to start was also investigated in the PREDICT trial in which Asian children of age 1–12 were included at a median age of 6.4 years, with only 6% in their second year of life.3,12,13 This trial didn’t show any difference between immediate ART initiation and deferring ART until either the CD4% was below 15% or any CDC category C event occurred - with respect to mortality and morbidity outcomes. The trial did however show better height gain for children who start ART immediately. However, the authors suggested that the study was underpowered to detect differences due to the lower than expected event rate. In Southern African children, a causal modelling study showed no mortality difference in 2–5 year old children for starting ART immediately versus starting ART when either the CD4 count falls below 750 cells/mm3 or the CD4% drops below 25%.14 In 2013, WHO guidelines were further updated to recommend ART initiation in all children less than 5 years. This change was mainly motivated by potential programmatic advantages, i.e. to provide simplified criteria for initiating ART and to bring young children into the health care system.
Thus, there still remain considerable evidence gaps: a comparison of different CD4 initiation criteria has never been explored for children aged 1–2 years. These children are known to have slower disease progression than infants, but also progress faster than older children and thus findings both from the CHER and PREDICT trial, as well from other recent analyses, may not apply to them.3,5 Moreover, it is of interest whether the evidence for children aged 2–5 years can be generalized to West African populations and whether the different growth response suggested by the PREDICT trial applies to these populations.
We thus used g-computation 14–16 to determine mortality and growth differences for different ART initiation strategies in young children from West and Southern Africa. We chose g-computation because it allows adjustment for time-varying confounders affected by prior treatment; in our data these are CD4 count, CD4%, and WHO stage (approximated by weight for age z-scores [WAZ]) which influence both ART initiation and our outcome measures. Traditional multivariate regression techniques may yield biased treatment effect estimates. An advantage of g-computation over competing methods is its suitability to compare dynamic intervention rules, its efficiency, and that it provides natural estimation of marginal effects.17
Our primary study aims were (i) to compare mortality and height outcomes for different CD4 based treatment initiation criteria (derived from selected CD4 criteria from WHO guidelines since 2006), (ii) to contrast mortality and growth of 1–2 year old children with older children, and (iii) to investigate the heterogeneity of results from Southern Africa and West Africa. All our estimates are based on the idealized conditions of regular visits (every 3 months) at which CD4 measurements are taken, and instantaneous treatment initiation if a treatment threshold has been reached.
METHODS
This study includes data of 16 cohorts from Côte d’Ivoire, Burkina Faso, Ghana, Senegal, Togo, South Africa, Malawi and Zimbabwe. All cohorts are part of either the IeDEA West Africa or IeDEA Southern Africa cohort collaboration. Both collaborations have been described elsewhere.18–21 In brief, data were collected at each facility as part of routine monitoring and were transferred to the coordinating data centres at Bordeaux University, France, University of Cape Town, South Africa, and University of Bern, Switzerland. All contributing sites obtained ethical approval from the relevant local institutions before submitting anonymized patient data to the collaboration. The data centres in Bordeaux, Bern, and Cape Town got ethical approval from the respective universities’ review boards to analyse this data.
The present study is limited to cohorts which routinely capture both pre-ART and post-ART data of HIV-infected children. All ART naïve children of age 12–59 months at enrollment with at least one visit before ART initiation and one follow-up visit were included (Figure 1).
Figure 1.
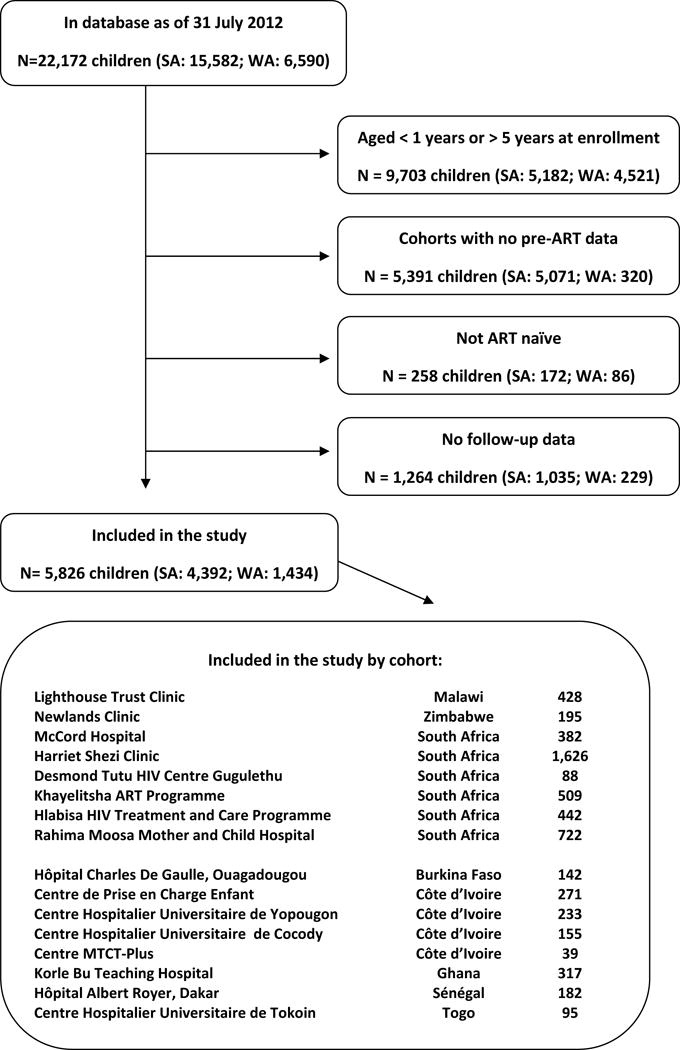
Flowchart: Selection of patients.
The analysis made use of children’s age at enrollment, their sex, treatment facility, date of ART initiation as well as CD4 count, CD4%, weight for age z-scores, and height for age z-scores, both at time of enrollment and during follow-up. All z-scores were calculated using WHO standards.22 We defined young children to be those who present at age 12–24 months (1–2 year age group), and old children to be those who present at age >24 months and ≤59 months (2–5 year age group). Follow-up data was evaluated one month after enrollment, three months after enrollment and then subsequently in 3-month intervals for a period of up to 3 years. If no data were available for a particular interval, the data were defined to be missing. Children were defined as being lost to follow-up (LTFU), and censored, if at the time of database closure they had no contact with their health care facility for at least 9 months since their last recorded visit. In a sensitivity analysis we censored children 9 months after having no contact with their health care facility, even if they re-entered care after 10 months or more.
We carried forward missing CD4 count, CD4%, weight- and height-for-age Z score follow-up data and used multiple imputation by means of the Expectation-Maximization-Bootstrap (EMB) algorithm 23 to deal with missing baseline data. The imputation model included all baseline variables, follow-up variables (including lagged and lead versions of them), death, a carry-forward indicator variable, and region, and also accounted for the longitudinal, possibly non-linear, structure of the data.
We summarized the data at time of enrollment, stratified by age and region, and during follow-up. Continuous data were described with medians (reported with first; third quartiles) and categorical data were summarized by proportions.
The primary analysis used g-computation to estimate cumulative mortality and growth (mean HAZ of children who are alive) during 3 years of follow-up for different interventions strategies: (a) immediate ART (b) delaying ART until CD4 fell below750 cells/mm3 or 25%, (c) delaying ART until CD4 fell below 350 cells/mm3 or 15%, or (d) no ART given. This analysis emulates the following clinical trial: HIV positive and ART-naïve children, aged 1–5 years, presenting at a health care facility for the first time, are randomly assigned one of the four treatment strategies (a)-(d). Each of the four arms is therefore differing by the CD4 thresholds used to determine the timing of ART initiation. Under full adherence to the regime, no administrative censoring, and no loss to follow-up, we can estimate the cumulative mortality at time t for each of the four regimes as well as the growth (mean height-for-age z score) of those children who survived until time t. We assume that CD4 count/% is measured and evaluated regularly because we are interested in the outcomes that would be observed if treatment strategies were followed; we therefore evaluate the outcomes under an ideal monitoring situation. We report cumulative mortality and growth for different follow-up times t, together with 95% bootstrap confidence intervals. We also report differences between the different interventions, regions, and age groups.
The mean height-for-age z score was chosen as the primary growth outcome to allow comparison with the PREDICT trial.13 Secondary growth outcomes are median height-for-age z score, proportion with height-for-age z score>−2, and cumulative incidence of attaining height-for-age z score>−2 before the competing event of death occurs. We estimate all growth outcomes under no administrative censoring, and no loss to follow-up, but allow for death of children, which means growth is estimated for children who survived until time t.
G-computation has been used before to determine the optimal timing of treatment initiation in adults.24,25 Our implementation of the g-computation algorithm is similar to the one described by Westreich et al.24 but differs slightly from this algorithm in that we use multiple imputation for baseline data and other variables, relevant to pediatric analyses, are included. To implement g-computation we had to model (at each time point t) the associations of CD4 count, CD4%, weight- and height-for age z scores, and death with disease progression history (CD4 count, CD4%, weight- and height-for age z scores at time t−1), baseline characteristics (CD4 count, CD4%, weight- and height-for-age z scores), demographics (age, sex, region), and the intervention (ART at times t−1 and t−2) using additive linear and logistic regression models. More details about our implementation are listed in eTextbox 2.
We conducted several sensitivity analyses: cumulative mortality was estimated (i) if only children with complete baseline data are included (ii) if missing follow-up data, as well as outcome data of lost children, is imputed (eTextbox 1). Growth was estimated (iii) if only children with complete baseline data are included, and (iv) under the assumption of no mortality in our population. We also estimated the outcomes and the confounders under the natural course, i.e. under no treatment intervention, and compared it to the observed data.16,25
In a secondary descriptive analysis, we estimated disease progression as the probability of falling below a CD4 value of 750 cells/mm3 or 25% for those children who were above this threshold at enrollment. Children LTFU, dead, or initiating ART were censored (first analysis) and treated as a competing risk (second analysis). Results were summarized using the Kaplan-Meier estimator and cumulative incidence curves 26 respectively.
RESULTS
Descriptive Results
Among 22,172 children in the database, 7,078 children were in the eligible age-range from cohorts that capture pre-ART data. After excluding 258 non-ART naïve children and 1,264 children with no follow-up, 5,826 were included in the analysis, of which 1,434 (24.6%) were from West Africa (Figure 1). Median (first; third quartile) follow-up was 27.4 (8.6; 35.0) months. Of 267 deaths, 158 (59.2%) occurred in Southern Africa and 58.8% within the first 6 months. Out of 1195 (20.5%) children LTFU 869 (72.3%) were from Southern Africa.
Patient characteristics are summarized in Table 1. At presentation, the median age was 2.6 (1.8; 3.8) years. The median CD4 count was 662 cells/mm3 (389; 1011), the median CD4 percent 16% (11%; 23%), and median height- and weight-for-age z scores were −2.5 (−3.5; −1.5) and −1.5 (−2.7; −0.6) respectively. Almost 75% of children started ART. Characteristics at enrollment were similar when comparing 1–2 year old with 2–5 year old children, though the latter had a slightly better weight- and height-for-age z scores profile. In both regions there was an improvement in median CD4 count/CD4%/weight- and height-for-age z scores over time, all outcomes being slightly better in Southern Africa than West Africa except for height-for-age z scores (eTable 1, eFigure 1). The increase of the mean height-for-age z score applied to children both on ART and not on ART (eFigure 1). The proportion of missing data at baseline varied from 18.4% for CD4 count to 40.3% for HAZ (Table 1). CD4 count/% was available every 3 months for 18%/15% of children; the median availability was every 6 months for CD4 count and every 8 months for CD4%. 19% [14%] of the 921 patients who had both a 3 monthly CD4 count and CD4% measurement started ART instantaneously given a 750/25% [350/15%] threshold.
Table 1.
Patient characteristics at first clinic visit, overall (Total) and stratified according to age and region. Available data are given in absolute numbers (percentages in brackets).
| 1–2 years | 2–5 years | Southern Africa | West Africa | Total | ||
|---|---|---|---|---|---|---|
| Sex | 1910 (100%) | 3916 (100%) | 4392 (100%) | 1434 (100%) | 5826 (100%) | |
| male | 954 (50.0%) | 2026 (51.7%) | 2222 (50.6%) | 758 (52.9%) | 2980 (51.2%) | |
| Age (in years) | 4392 (100%) | 1434 (100%) | 5826 (100%) | |||
| Median (1st; 3rd quartile) | 2.6 (1.7 ; 3.7) | 2.7 (1.8 ; 3.8) | 2.6 (1.8 ; 3.8) | |||
| CD4 count | 1545 (80.9%) | 3208 (81.9%) | 3692 (84.1%) | 1061 (74.0%) | 4753 (81.6%) | |
| Median (1st; 3rd quartile) | 790 (475 ; 1196) | 607 (365 ; 912) | 646 (380 ; 984) | 719 (433 ; 1081) | 662 (389 ; 1011) | |
| >750 | 818 (52.9%) | 1165 (36.3%) | 1486 (40.3%) | 497 (46.8%) | 1983 (41.7%) | |
| CD4% | 1384 (72.5%) | 2775 (70.9%) | 3409 (77.6%) | 750 (52.3%) | 4159 (71.4%) | |
| Median (1st; 3rd quartile) | 16% (11% ; 22%) | 16% (10% ; 23%) | 16% (11% ; 23%) | 16% (10% ; 22%) | 16% (11% ; 23%) | |
| >25% | 242 (17.5%) | 507 (18.3%) | 633 (18.6%) | 116 (15.5%) | 749 (18.0%) | |
| WAZ | 1446 (75.7%) | 2914 (74.4%) | 3766 (85.8%) | 594 (41.4%) | 4360 (74.8%) | |
| Median (1st; 3rd quartile) | −1.9 (−3.2 ; −0.8) | −1.4 (−2.4 ; −0.6) | −1.5 (−2.6 ; −0.6) | −1.9 (−3.2 ; −0.9) | −1.5 (−2.7 ; −0.6) | |
| <−2 | 700 (48.4%) | 981 (33.7%) | 1397 (37.1%) | 284 (47.8%) | 1681 (38.6%) | |
| HAZ | 1092 (57.2%) | 2387 (61.0%) | 2972 (67.7%) | 507 (35.4%) | 3479 (59.7%) | |
| Median (1st; 3rd quartile) | −2.6 (−3.8 ; −1.5) | −2.5 (−3.4 ; −1.5) | −2.6 (−3.6 ; −1.6) | −2.1 (−3.1 ; −1.0) | −2.5 (−3.5 ; −1.5) | |
| <−2 | 703 (64.4%) | 1504 (63.0%) | 1929 (64.9%) | 278 (54.8%) | 2207 (63.4%) | |
| Region | 1910 (100%) | 3916 (100%) | 5826 (100%) | |||
| West Africa | 452 (23.7%) | 982 (25.1%) | 1434 (24.6%) | |||
| Ever started ART | 1910 (100%) | 3916 (100%) | 4392 (100%) | 1434 (100%) | 5826 (100%) | |
| yes | 1421 (74.4%) | 2936 (75.0%) | 3318 (75.6%) | 1039 (72.5%) | 4357 (74.8%) | |
Progression to CD4 threshold
The estimated proportion of children who progressed to below the threshold of 750 cells/mm3 or 25% was similar in Southern and West Africa throughout follow-up, irrespective of whether a competing risk approach was used or not (Figure 2). After 3 years, 71.6% (95% CI: 64.8%; 78.1%) of Southern African children and 74.9% (95% CI: 59.5%; 87.9%) of West African children were estimated to have progressed below the threshold (Kaplan-Meier estimator). The estimates obtained from the cumulative incidence curves were slightly higher (75.7% (69.7%; 81.9%) and 83.6% (68.1%; 94.4%) respectively).
Figure 2.
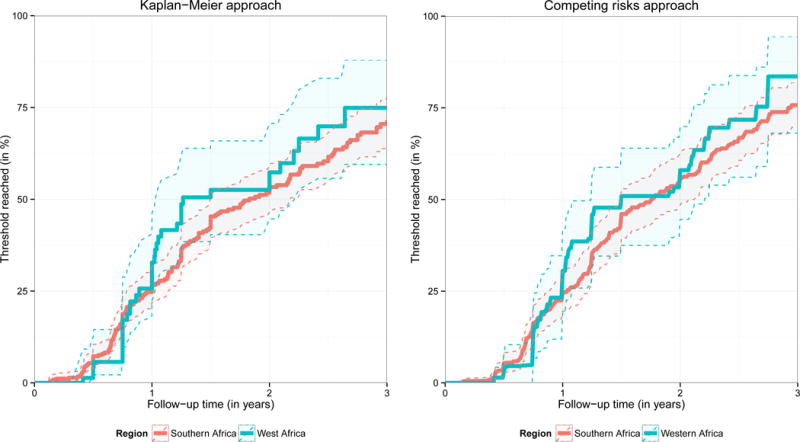
Estimated probability of falling below a CD4 count of 750 cells/mm3 or a CD4 percentage of 25%. (A) via the Kaplan-Meier estimator: this figure is based on 613 children (514 from Southern Africa, 99 from West Africa) presenting with a CD4 count of 750 cells/mm3 or above and a CD4% of 25% or above. Only pre-ART follow-up is considered and lost or dead children were censored at the time of the respective event. (B) ART initiation, death, and loss to follow-up (LTFU) were treated as competing risks. The probability of falling below the threshold was estimated via the cumulative incidence of falling below the threshold before any other event occurred divided by one minus the probability that any other event occurred before the threshold was reached. 95% confidence intervals were obtained via bootstrapping and are visualised via the shaded area.
Mortality Analysis
The estimated cumulative mortality for different treatment strategies is summarized in Figure 3 (top left) and eTable2. There is a trend towards higher mortality associated with starting ART at lower CD4 thresholds during the whole follow-up period. After 3 years of follow-up mortality for the different strategies was estimated to be 8.8% (7.7%; 11.1%) (no ART), 5.7% (5.0%; 6.6%) (ART if CD4 <350/15%), 5.0% (4.3%; 5.9%) (ART if CD4 <750/25%), and 4.8% (4.3%, 5.9%) (immediate ART). The estimated mortality difference between the latter two strategies was 0.2% (−0.2%; 0.3%) after 3 years and 0.9% (0.3%; 1.2%) between immediate ART and giving ART if CD4 <350/15% (Figure 3, top right and eTable 2a). The trends can be seen in all age groups and regions (eTable2). Mortality after 3 years was estimated to be higher among 1–2 year olds compared to older children (mortality difference [MD] for immediate ART 1.3% (0.5%; 2.8%), Figure 3, bottom left, eTable 2b) and in West Africa compared to Southern Africa (MD for immediate ART: 4.6% (2.9%; 5.9%), Figure 3, bottom right and eTable2). The mortality differences between age groups and regions are mostly driven by the first three months after the first visit and remain reasonably stable thereafter (Figure 3, bottom panel). The sensitivity analyses led to similar conclusions when comparing interventions, age groups, and regions (eFigure2, eFigure 3). Mortality was somewhat greater when using the extended imputation approach, and slightly smaller when restricting the analysis to children with complete baseline data.
Figure 3.
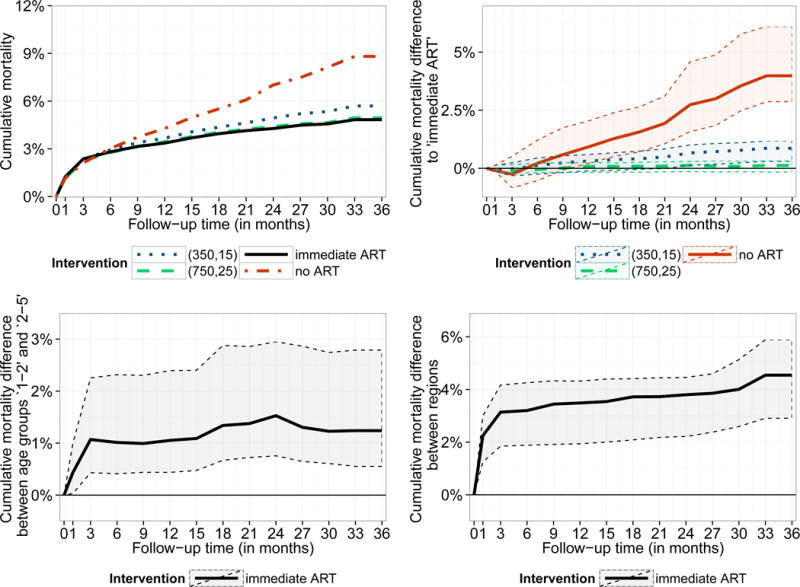
Estimated cumulative mortality by intervention strategy (top left) and differences between these intervention strategies (top right). For the intervention ‘immediate ART’ cumulative mortality differences are displayed for both comparing the two different age groups and regions (bottom panel). Results are based on g-computation and 95% bootstrap confidence intervals are represented by the shaded area.
Growth Analysis
Figure 4 (top left) demonstrates that the mean HAZ of surviving patients after 3 years of follow-up ranges between −1.92 (−2.09; −1.72) (no ART) and −1.73 (−1.88; −1.50) (immediate ART). In comparison to immediate ART, the differences in mean HAZ after 3 years were estimated as −0.02 (−0.04; 0.01) (CD4 count/% threshold of 750/25%), −0.08 (−0.10; −0.05) (threshold: 350/15%) and −0.19 (−0.25; −0.16) (no ART) (Figure 4, top right). Using the median HAZ, the probability of HAZ>−2 and the cumulative incidence of HAZ>−2 as outcome yield the same conclusions (eFigure 4, eTable 3). The mean HAZ is generally lower in younger children and in South Africa; the differences between the age groups are however reasonably stable over time (Figure 4, bottom panel, eFigure 5). The sensitivity analyses confirm the above findings (eFigure 6, eFigure 7).
Figure 4.
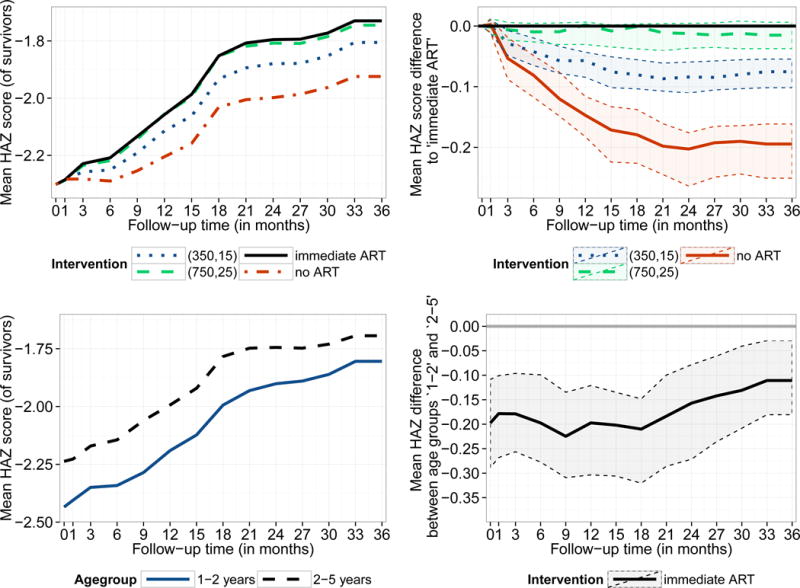
Estimated mean height-for-age z-score by intervention strategy (for survivors at the respective time point1, top left) and differences between these intervention strategies (top right). For the intervention ‘immediate ART’ the mean height-for-age z-score for both age groups as well as the difference between them are displayed (bottom panel). Results are based on g-computation and 95% bootstrap confidence intervals are represented by the shaded area.
The data characteristics are overall similar when comparing the observed data with the data simulated with g-computation under the natural course scenario, i.e. under no intervention, though for the CD4 measurements there are some deviations after 1 year of follow-up (eFigure 8). A different definition of LTFU led to overall similar results (eFigure9).
DISCUSSION
Statement of principal findings
We found overall lower mortality and better growth when starting ART earlier in children aged 1–5. These differences were very small when comparing immediate ART initiation with deferring ART until the CD4 threshold of 750/25% is reached, but clearer when comparing it with the CD4 threshold of 350/15%. Our findings were consistent over age groups and regions, but mortality was estimated to be lower, and growth to be faster, in children aged 2–5 and in Southern Africa.
Strengths of the study
This is the first implementation of g-computation to estimate growth differences associated with different treatment initiation rules. Consistent findings from the Southern and West Africa regions with differences in background morbidity, access and standard of HIV care, patient populations, and training possibilities are reassuring in terms of generalizability of our results. Our findings complement other studies which either focused on other age groups7,14 or were underpowered13.
Limitations
The outcomes of children lost to follow-up were not known. While censoring by drop out can be handled using g-computation, and we conducted sensitivity analyses using multiple imputation, it may be possible that those defined to be lost have a particular high risk of being dead. In some settings, this is known to be true for adults; 27–29 however, in children less is known about those lost to follow-up because reasons for missing an appointment may relate to the caregiver’s work responsibilities, family situations, economic opportunities, and own health status 30. Studies that trace lost children or link them to vital registries are needed to gain more knowledge about this group. While it is possible that the deviations between observed CD4 data and the data generated under the natural course scenario (eFigure 8) are due to informative censoring, unmeasured confounding related to clinical events not captured by weight-for-age z-score data (i.e. encephalopathy or HIV-associated nephropathy), or model misspecification, offer alternative explanations.
Our study requires children to have at least one follow-up visit. Children who were excluded because of no follow-up data had higher CD4 counts (median [first; third quartile]: 742 [422; 1220]) and higher CD4% (20 [13;28]) but lower weight- and height-for-age z-score values (−2.3 [−3.7; −0.9] and −2.8 [−3.8; −1.5] respectively). This means that mortality at the population level could potentially differ from our estimates.
We assume CD4 count/% to be measured 3 monthly and antiretroviral treatment to be started instantaneously after patients become eligible under the respective treatment strategy. Our estimates are therefore obtained under idealized conditions and may not be directly applicable to existing conditions in sub-Saharan Africa, i.e. if CD4 is performed less frequently or initiation of ART is not instantaneous after dropping below the threshold, it is possible that mortality and growth differences between immediate and deferred ART would be different from our estimates. However, it is noteworthy that both the PREDICT and the CHER trial scheduled visits no more than 3 months apart.
Other limitations relate to the lack of long-term outcome data and therefore the possibility of examining effects of toxicities and drug resistance. Also, missing data on first line regimens and differences in drug use between countries make it difficult to evaluate growth and mortality outcomes stratified by ART regimen. It may be possible that children on nevirapine show better growth outcomes compared to children using lopinavir, but also higher virological failure which could result in higher mortality 31.
Results in context
While we found a trend towards higher mortality being associated with starting ART at lower CD4 thresholds, differences between the criteria of starting ART immediately and delaying until CD4<750/25% were negligible, for all follow-up times and across regions and age groups. In line with previous reports13,14 this suggests that the change in WHO guidelines in 2013 may neither result in increased nor decreased mortality in young children. However, estimated mortality was higher, and growth slower, when starting ART at the 350/15% threshold, confirming that later ART initiation, as recommended in 2006, may have consequences in terms of both mortality and growth.
In line with the results of the PREDICT trial, where children were enrolled with higher HAZ than in our study (mean HAZ −1.7 vs. −2.5), we found a better growth response related to early ART initiation. We could however show no difference related to the criteria of starting ART immediately versus delaying until the threshold of 750/25% is reached. An important consideration regarding interpretation and understanding of our growth results relates to the fact that at different time points and for different interventions a different number of survivors remain, and thus comparisons are difficult. However, our results from the competing risk and sensitivity analyses (eTable 3, eFigure 7) address this concern and confirm the overall findings of the mean height-for-age z-score results. Moreover, our results can be considered as conservative estimates of the effect of earlier ART initiation: the number of survivors at lower ART initiation thresholds is overall lower with some of the sicker patients, with lowest height-for-age z score, thus excluded from the mean score when compared to higher thresholds; therefore true differences between initiation criteria for any particular group of survivors might be larger than reported by us.
In our data mortality was estimated to be higher in West Africa when compared to Southern Africa. This may relate to higher malnutrition in West Africa, a different background of co-infections such as malaria, difficult access to care, different ART monitoring, and the role of stigma 32,33. However, these differences were largely driven by differences during the first 3 months after enrollment. As indicated earlier, it might be possible that the high proportion of lost children in the first 3 months in Southern Africa includes a substantial proportion of children who died. It is still possible that some of the differences between regions can be attributed to under-ascertained mortality in Southern Africa.
Mortality was estimated to be higher, and growth slower, in children aged 1–2 compared to older children. This mainly reflects the differences at presentation: the group of children aged 1–2 contains many children who have probably been infected in utero or at birth. These children tend to have more severe disease, with a worse survival prognosis: if a child is not diagnosed with HIV in early infancy, it may only be identified too late, i.e. when caregivers arrive at a health care facility with a very sick child. These children have a high risk of death before or shortly after presentation. The age group of children aged 2–5 contains fewer of these children because these children are more long-term survivors when they are 2 or older than earlier. In addition, even in the absence of HIV, mortality is highest in infants and declines with increasing age during childhood.
Further considerations and future directions
Our results suggest no negative consequences regarding mortality and growth response when starting ART at the earliest presentation to health care services in young children aged 1–5. There are however other relevant considerations for determining the optimal time of treatment initiation: in settings with limited resources and few trained health care workers the allocation of resources needs to be considered carefully. Rapid ART initiation should not happen at the expense of neglecting early infant diagnosis and withholding care from the most vulnerable children. However, only about 11% of the children in our data presented with a CD4 count>750 cells/mm3 and CD4%>25% and about 75% of those progressed to below this threshold within 3 years. This suggests that the additional burden of early treatment initiation may be moderate, although probably underestimated by us because our cohorts may not be representative of all HIV infected children between 1–5 years of age. Antiretroviral treatment also implies lifelong therapy and therefore early treatment goes along with longer exposure to the risks of non-adherence, toxicity, and resistance. It remains important to identify a caregiver who understands the implications and responsibilities of starting ART. Moreover, there may be non-identified long term risks and changing WHO guidelines back to delayed treatment may not be feasible anymore.
Nevertheless, recommending treatment initiation in all young children regardless of their immunological and clinical stage, may improve access to care, simplify pediatric treatment and facilitate expansion of ART coverage. Given the rapid disease progression in young children, using CD4 criteria may not delay the onset of therapy very much and risk children of dropping out of health care. Moreover, regular CD4 measurements are not available in all resource-limited settings. Early treatment initiation may also be beneficial for immune recovery and neurodevelopment.
Conclusions
In conclusion, early treatment initiation yields better growth and mortality outcomes in children aged 1 to 5 from West and Southern Africa, though differences between immediate ART initiation and delaying until CD4<750/25% are negligible. Younger children had worse outcomes in our study, but future studies need to confirm this and address programmatic and long-term implications and challenges of early treatment initiation to improve their survival.
Supplementary Material
Acknowledgments
Computations were performed using facilities provided by the University of Cape Town’s ICTS High Performance Computing team. We would like to thank Martina Penazzato (WHO), Lulu Muhe Mussa (WHO) and Shaffiq Essajee (Clinton Foundation) for their guidance on the initial design of the project. The authors are also grateful to all patients’ families and staff at the HIV care programmes included in this analysis and to the staff at the data centres. We would also like to acknowledge the IeDEA-WA and IeDEA-SA steering groups listed below.
IeDEA-SA Steering Group: Frank Tanser, Africa Centre for Health and Population Studies, University of Kwazulu-Natal, Somkhele, South Africa; Christopher Hoffmann, Aurum Institute for Health Research, Johannesburg, South Africa; Benjamin Chi, Centre for Infectious Disease Research in Zambia, Lusaka, Zambia; Denise Naniche, Centro de Investigação em Saúde de Manhiça, Manhiça, Mozambique; Robin Wood, Desmond Tutu HIV Centre (Gugulethu and Masiphumelele clinics), Cape Town, South Africa; Diana Dickinson, Independent Surgery, Gaborone, Botswana; Kathryn Stinson, Khayelitsha ART Programme and Médecins Sans Frontières, Cape Town, South Africa; Geoffrey Fatti, Kheth’Impilo Programme, South Africa; Sam Phiri, Lighthouse Trust Clinic, Lilongwe, Malawi; Janet Giddy, McCord Hospital, Durban, South Africa; Maureen Wellington, Newlands Clinic, Harare, Zimbabwe; Kennedy Malisita, Queen Elizabeth Hospital, Blantyre, Malawi; Brian Eley, Red Cross War Memorial Children’s Hospital and School of Child and Adolescent Health, University of Cape Town, Cape Town, South Africa; Jara Llenas, SolidarMed SMART Programme, Pemba Region, Mozambique; Christiane Fritz, SolidarMed SMART Programme, Masvingo, Zimbabwe; Matthew Fox and Mhairi Maskew, Themba Lethu Clinic, Johannesburg, South Africa; Hans Prozesky, Tygerberg Academic Hospital, Stellenbosch, South Africa; Karl Technau, Empilweni Clinic, Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa; Shobna Sawry, Harriet Shezi Children’s Clinic, Chris Hani Baragwanath Hospital, Soweto, South Africa.
IeDEA-WA Paediatric Group: (*members of the Steering Committee, §members of the Executive Committee): Benin, Cotonou: Pediatrics: Sikiratou Koumakpaï*§, Florence Alihonou, Marcelline d’Almeida, Irvine Hodonou, Ghislaine Hounhoui, Gracien Sagbo, Leïla Tossa-Bagnan, Herman Adjide (CNHU Hubert Maga). Burkina Faso: Pediatrics: Diarra Yé*, Fla Kouéta, Sylvie Ouedraogo, Rasmata Ouédraogo, William Hiembo, Mady Gansonré (CH Charles de Gaulle, Ouagadougou). Côte d’Ivoire, Abidjan: Pediatrics: Koffi Ladji Issouf, Jean-Claude Kouakou, Marie-Sylvie N’Gbeche*, (ACONDA-CePReF); Touré Pety*, Divine Avit-Edi (ACONDA-MTCT-Plus); Kouadio Kouakou*, Magloire Moh, Valérie Andoblé Yao (CIRBA); Madeleine Amorissani Folquet*, Marie-Evelyne Dainguy, Cyrille Kouakou, Véronique Tanoh Méa-Assande, Gladys Oka-Berete, Nathalie Zobo, Patrick Acquah, Marie-Berthe Kokora (CHU Cocody); Tanoh François Eboua*, Marguerite Timité-Konan, Lucrèce Diecket Ahoussou, Julie Kebé Assouan, Mabéa Flora Sami, Clémence Kouadio (CHU Yopougon). Ghana, Accra: Pediatrics: Lorna Renner*§, Bamenla Goka, Jennifer Welbeck, Adziri Sackey, Seth Ntiri Owiafe (Korle Bu TH). Mali, Bamako: Pediatrics: Fatoumata Dicko*, Mariam Sylla, Alima Berthé, Hadizatou Coulibaly Traoré, Anta Koïta, Niaboula Koné, Clémentine N’Diaye, Safiatou Touré Coulibaly, Mamadou Traoré, Naïchata Traoré (CH Gabriel Toure). Senegal, Dakar: Pediatrics: Haby Signate Sy*, Abou Ba, Aida Diagne, Hélène Dior, Malick Faye, Ramatoulaye Diagne Gueye, Aminata Diack Mbaye (CH Albert Royer). Togo, Lomé: Pediatrics: Koko Lawson-Evi*§, Yawo Atakouma, Elom Takassi, Améyo Djeha, Ayoko Ephoévi-gah, Sherifa El-Hadj Djibril (CHU Tokoin/Sylvanus Olympio). Executive Committee*: François Dabis (Principal Investigator, Bordeaux, France), Emmanuel Bissagnene (Co-Principal Investigator, Abidjan, Côte d’Ivoire), Elise Arrivé (Bordeaux, France), Patrick Coffie (Abidjan, Côte d’Ivoire), Didier Ekouevi (Abidjan, Côte d’Ivoire), Antoine Jaquet (Bordeaux, France), Valériane Leroy (Bordeaux, France), Annie J Sasco (Bordeaux, France). Operational and Statistical Team: Jean-Claude Azani (Abidjan, Côte d’Ivoire), Eric Balestre (Bordeaux, France), Serge Bessekon (Abidjan, Côte d’Ivoire), Sophie Karcher (Bordeaux, France), Jules Mahan Gonsan (Abidjan, Côte d’Ivoire), Jérôme Le Carrou (Bordeaux, France), Séverin Lenaud (Abidjan, Côte d’Ivoire), Célestin Nchot (Abidjan, Côte d’Ivoire), Karen Malateste (Bordeaux, France), Amon Roseamonde Yao (Abidjan, Côte d’Ivoire). Administrative Team: Abdoulaye Cissé (Abidjan, Côte d’Ivoire), Alexandra Doring§ (Bordeaux, France), Adrienne Kouakou (Abidjan, Côte d’Ivoire), Guy Gneppa (Abidjan, Côte d’Ivoire), Elodie Rabourdin (Bordeaux, France), Jean Rivenc (Pessac, France).
Source of Funding
This work was supported by the US National Institute of Allergy and Infectious Diseases (NIAID) through the International epidemiological Databases to Evaluate AIDS, Southern Africa (IeDEA-SA) and West Africa, grant numbers 5U01AI069924-05 and U01AI069919, the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD), The National Cancer Institute (NCI), and the World Health Organization (WHO). The opinions expressed herein are those of the authors and do not necessarily reflect the views of any of the funders.
Footnotes
Conflicts of Interest
The National Institutes of Health, WHO, NIAID, NCI, and NICHD had no role in data collection and analysis, decision to publish, and preparation of the manuscript.
Note: the number of survivors is different for each time point and strategy. Consult the discussion for more insight.
Bibliography
- 1.UNAIDS. Report on the global AIDS epidemic 2013. Geneva: 2013. [Google Scholar]
- 2.Prendergast AJ, Penazzato M, Cotton M, et al. Treatment of young children with HIV infection: using evidence to inform policymakers. Plos Med. 2012;9(7):e1001273. doi: 10.1371/journal.pmed.1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puthanakit T, Bunupuradah T. Early versus deferred antiretroviral therapy in children in low-income and middle-income countries. Current Opinions in HIV/AIDS. 2010;5(1):12–17. doi: 10.1097/COH.0b013e3283339b27. [DOI] [PubMed] [Google Scholar]
- 4.Siegfried N, Davies MA, Penazzato M, Muhe LM, Egger M. Optimal time for initiating antiretroviral therapy (ART) in HIV-infected, treatment-naive children aged 2 to 5 years old. Cochrane Database Syst Rev. 2013;10:CD010309. doi: 10.1002/14651858.CD010309.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turkova A, Webb RH, Lyall H. When to start, what to start and other treatment controversies in pediatric HIV infection. Paediatr Drugs. 2012;14(6):361–376. doi: 10.2165/11599640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Schomaker M. Implications of causal modelling studies on the question of when to start antiretroviral treatment in young children. SACEMA Quarterly. 2014 Nov; http://sacemaquarterly.com/2014/11.
- 7.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. New England Journal of Medicine. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross Continents Collaboration for Kids (3Cs4kids) Analysis and Writing Committee. Markers for predicting mortality in untreated HIV-infected children in resource-limited settings: a meta-analysis. AIDS. 2008;22(1):97–105. doi: 10.1097/01.aids.0000302262.51286.a5. [DOI] [PubMed] [Google Scholar]
- 9.Dunn D, HIV Paediatric Prognostic Markers Collaborative Study Group Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet. 2003;362(9396):1605–1611. doi: 10.1016/s0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 10.Patel K, Hernan MA, Williams PL, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(11):1751–1760. doi: 10.1086/587900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch SB, Gibb D. When should children with HIV infection be started on antiretroviral therapy? Plos Med. 2008;5(3):e73. doi: 10.1371/journal.pmed.0050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive Function and Neurodevelopmental Outcomes in HIV-infected Children Older Than 1 Year of Age Randomized to Early Versus Deferred Antiretroviral Therapy: The PREDICT Neurodevelopmental Study. Pediatr Infect Dis J. 2013;32(5):501–508. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puthanakit T, Saphonn V, Ananworanich J, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infectious Diseases. 2012;12(12):933–941. doi: 10.1016/S1473-3099(12)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schomaker M, Egger M, Ndirangu J, et al. When to start antiretroviral therapy in children aged 2–5 years: a collaborative causal modelling analysis of cohort studies from southern Africa. Plos Med. 2013;10(11):e1001555. doi: 10.1371/journal.pmed.1001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel RM, Cousens SN, De Stavola BL, Kenward MG, Sterne JA. Methods for dealing with time-dependent confounding. Stat Med. 2013;32(9):1584–1618. doi: 10.1002/sim.5686. [DOI] [PubMed] [Google Scholar]
- 16.Daniel RM, De Stavola BL, Cousens SN. G- formula: Estimating causal effects in the presence of time-varying confounding or mediation using the g-computation formula. The Stata Journal. 2011;11(4):479–517. [Google Scholar]
- 17.Robins J, Hernan MA, Siebert U. Comparative quantication of health risks: global and regional burden of disease attributable to selected major risk factors. In: Ezzati M, Murray C, Lopez A, editors. Effects of multiple interventions. World Health Organization; 2004. pp. 2191–2230. [Google Scholar]
- 18.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41(5):1256–1264. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenner L, Brinkhof MWG, Keiser O, et al. Early Mortality and Loss to Follow-up in HIV-Infected Children Starting Antiretroviral Therapy in Southern Africa. Jaids-J Acq Imm Def. 2010;54(5):524–532. doi: 10.1097/QAI.0b013e3181e0c4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekouevi DK, Azondekon A, Dicko F, et al. 12-month mortality and loss-to-program in antiretroviral-treated children: The IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000–2008. Bmc Public Health. 2011;11:519. doi: 10.1186/1471-2458-11-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group IPW. A survey ofpaediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa–the International epidemiologic Databases to Evaluate AIDS (IeDEA) Journal of the International AIDS Society. 2013;16(1):17998. doi: 10.7448/IAS.16.1.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. The WHO Child Growth Standards. 2006 http://www.who.int/childgrowth/en/. Accessed 23-11-2008.
- 23.Honaker J, King G. What to do about missing values in time-series cross-section data? Am J Polit Sci. 2010;54:561–581. [Google Scholar]
- 24.Westreich D, Cole SR, Young JG, et al. The parametric g-formula to estimate the effect of highly active antiretroviral therapy on incident AIDS or death. Stat Med. 2012;31(18):2000–2009. doi: 10.1002/sim.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young JG, Cain LE, Robins JM, O’Reilly EJ, Hernan MA. Comparative effectiveness of dynamic treatment regimes: an application of the parametric g-formula. Stat Biosci. 2011;3(1):119–143. doi: 10.1007/s12561-011-9040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 27.Kiragga AN, Castelnuovo B, Musomba R, et al. Comparison of methods for correction of mortality estimates for loss to follow-up after ART initiation: a case of the Infectious Diseases Institute, Uganda. Plos One. 2013;8(12):e83524. doi: 10.1371/journal.pone.0083524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schomaker M, Gsponer T, Estill J, Fox M, Boulle A. Non-ignorable loss to follow-up: correcting mortality estimates based on additional outcome ascertainment. Stat Med. 2014;33(1):129–142. doi: 10.1002/sim.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkhof M, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. Plos One. 2009;4:e5790–e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bwirire LD, Fitzgerald M, Zachariah R, et al. Reasons for loss to follow-up among mothers registered in a prevention-of-mother-to-child transmission program in rural Malawi. Trans R Soc Trop Med Hyg. 2008;102(12):1195–1200. doi: 10.1016/j.trstmh.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012 Jun 21;366(25):2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jesson J, Koumakpai A, Dior H, Amorissani-Folquet M, Kouéta F, Aka A. Effect of age at antiretroviral treatment initiation on growth catch up within the first 24 months of treatment among HIV-infected children in the IeDEA West African paediatric cohort. Pediatric Infectious Disease Journal. 2014 doi: 10.1097/INF.0000000000000734. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. World Malaria Report. Geneva: World Health Organization; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


