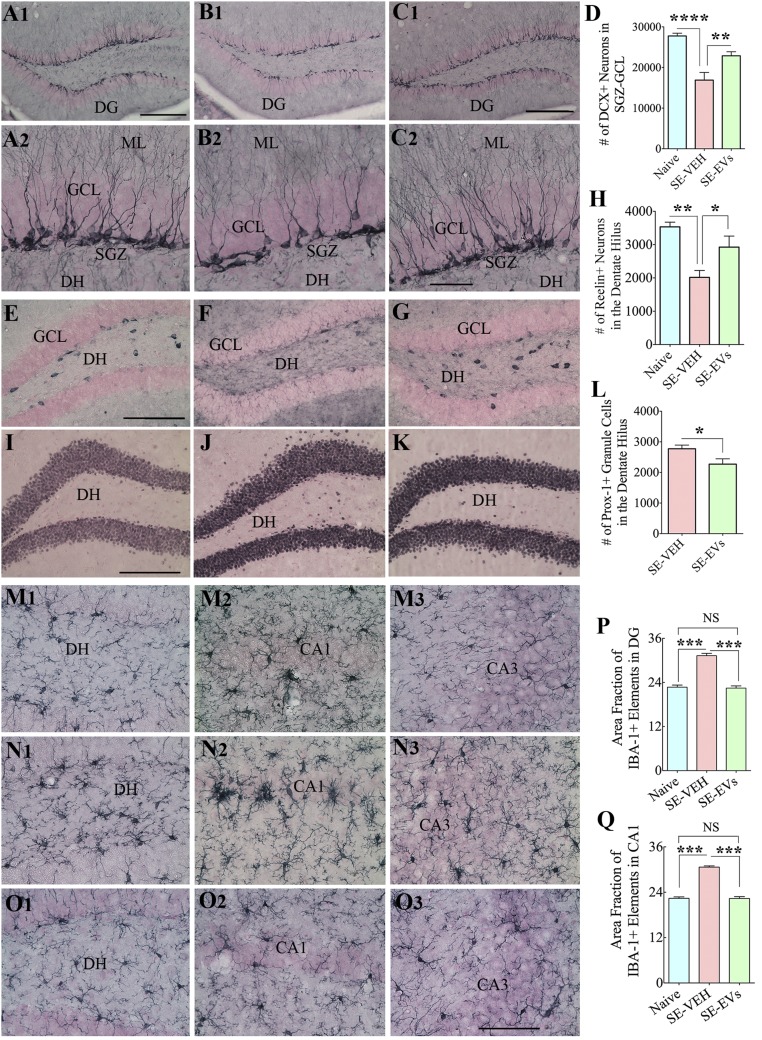
Fig. 7.
IN administration of A1-exosomes 2 h after SE restrains multiple adverse changes that are typically seen in the chronic phase after SE. In comparison with naive control animals (A1, A2, E, I, and M1–M3), animals in the SE-VEH group showed waning of hippocampal neurogenesis [doublecortin (DCX) immunostaining; B1 and B2], loss of reelin+ interneurons in the DG (F), aberrant migration of newly born prox-1+ granule cells into the DH (J), and persistent hippocampal inflammation (with increased density and hypertrophy of IBA-1+ microglia; N1–N3). In animals in the SE-EVs group, the extent of neurogenesis (C1 and C2), the survival of reelin+ interneurons (G), and the morphology and density of IBA-1+ microglia (O1–O3) were comparable to those observed in naive control animals (A1, A2, E, I, and M1–M3). In addition, aberrant migration of newly born cells into the DH was reduced (K) in these animals. ML, molecular layer. Bar charts compare numbers of DCX+ newly born neurons in the SGZ-GCL (D), reelin+ interneurons in the DH (H), numbers of prox-1+ newly born granule cells in the DH (L), and IBA-1+ microglia in the DG (P) and the CA1 subfield (Q) between groups. Note that the extent of neurogenesis (D) and numbers of reelin+ interneurons (H) and IBA-1+ structures (P and Q) in SE-EVs group animals were comparable to those seen in naive control animals. In addition, SE-EVs animals showed reduced numbers of prox-1+ cells in the DH (L), implying a reduced abnormal migration of newly born granule cells with A1-exosome treatment after SE (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001). (Scale bars: A1, B1, and C1, 200 µm; A2, B2, and C2, 50 µm; E–G and I–K, 200 µm; M1–O3, 100 µm.)