Abstract
PURPOSE
Transjugular intrahepatic portosystemic shunt (TIPS) creation is used to treat portal hypertension complications. Often the most challenging and time-consuming step in the procedure is the portal vein (PV) puncture. TIPS procedures are associated with prolonged fluoroscopy time and high patient radiation exposures. We measured the impact of transabdominal ultrasound guidance for PV puncture on duration of fluoroscopy time and dose.
METHODS
We retrospectively analyzed the radiation dose for all TIPS performed over a four-year period with transabdominal ultrasound guidance for PV puncture (n=212, with 210 performed successfully and data available for 206); fluoroscopy time, dose area product (DAP) and skin dose were recorded.
RESULTS
Mean fluoroscopy time was 12 min 9 s (SD, ±14 min 38 s), mean DAP was 40.3±73.1 Gy·cm2, and mean skin dose was 404.3±464.8 mGy.
CONCLUSION
Our results demonstrate that ultrasound-guided PV puncture results in low fluoroscopy times and radiation doses, which are markedly lower than the only published dose reference levels.
Transjugular intrahepatic portosystemic shunt (TIPS) formation is an effective treatment for the complications of portal hypertension in both cirrhotic and noncirrhotic patients. Primarily this relates to treatment of refractory variceal hemorrhage (1) but is also used in secondary prevention of variceal hemorrhage, and to treat refractory ascites, Budd-Chiari syndrome, hepatic hydrothorax (2), and occasionally as a therapy for portal vein thrombosis (3).
The most technically demanding and usually the limiting step of the procedure is the portal vein (PV) puncture (4). This is associated with a number of potential complications including capsular laceration during wedged venography (5), gallbladder puncture, intra-abdominal hemorrhage following extrahepatic puncture of the main portal vein (6) or transcapsular puncture. A variety of techniques have been described for PV access. Early TIPS used the target of a Dormia basket in the right PV that had been inserted percutaneously (7). As knowledge of the procedure and relevant anatomical considerations increased (8), “blind” fluoroscopic guidance of the needle was widely used. A wide range of techniques have emerged to guide PV puncture (9) including wedged venography with carbon dioxide or contrast (4), the latter with success rates of approximately 90% (9). Other methods include arterial portography, the “gun-sight” technique (10), hybrid magnetic resonance imaging (MRI) and fluoroscopy (11), cone beam computed tomography (CT) (12), and intravascular ultrasound (13). Transabdominal ultrasound guidance offers a noninvasive method, which has infrequently been reported in the literature (14–17), and despite its advantages is not widely used in Europe (4).
TIPS is one of the most complex interventional radiology procedures with prolonged fluoroscopy times and numerous cases of radiation-induced skin injury (18–20). Studies have also demonstrated high operator doses (21). In a series of 18 cases reported by Hidajat et al. (21), fluoroscopy time was 77.8±66.3 min and dose area product (or kerma area product) (DAP) was 446±280 Gy·cm2, although no details were given about the mode of guidance for PV puncture.
The multicenter Radiation Doses in Interventional Radiology Procedures (RAD-IR) (21) recorded radiation doses for a variety of interventional radiology procedures from seven academic centers in the United States, measuring fluoroscopy time, DAP, and cumulative (i.e., skin) dose. A total of 135 TIPS cases were accrued within the 12-year time period; with mean fluoroscopy time 38.7 min (range, 3.5–153 min; 95% CI, 34.2–43.3 min); DAP 335 Gy·cm2 (range, 14–1364 Gy·cm2; 95% CI, 291–380 Gy·cm2) and reference dose (or cumulative/skin dose) 2039 mGy (range, 104–7160 mGy; 95% CI, 1760–2317 mGy) (22). In 2009 these data were used to determine dose reference levels (DRLs) for the United States: fluoroscopy time, ≤60 min; DAP, ≤525 Gy·cm2 (23); and reference dose, ≤ 3000 mGy. There is a paucity of European DRLs data for TIPS (24). Currently Miraglia et al. (16) are the only European center to have published local DRLs for TIPS creation. Their reference levels were based on the 75th percentile and therefore suggested that a DAP of less than 150 Gy·cm2 and a fluoroscopy time of less than 25 min were acceptable. This is significantly less than the DRL reported by Miller et al. (23).
We describe the experience from our institution where TIPS has been performed using transabdominal ultrasound-guidance to direct the PV puncture since March 2011, recording the impact on fluoroscopy times and radiation doses with comparison to published DRLs.
Methods
All TIPS procedures performed in our institution between March 19, 2011 and March 19, 2015 were retrospectively reviewed from the picture archiving and communication system (PACS) and radiology information system (RIS) and indication for TIPS, fluoroscopy time, DAP (Gy·cm2) and skin dose (mGy) were recorded; with the dosimetry details acquired from the integrated dosimetry within the fluoroscopy machine. The research was performed according to the World Medical Association Declaration of Helsinki. Ethical approval was not deemed necessary, as this was a retrospective review of established practice in our institution. No specific informed consent was obtained as this was a retrospective analysis of a previously compiled anonymized database.
Patients
A total of 212 procedures were performed. The indications for creation of TIPS are listed in Table 1. In two patients TIPS could not be performed due to occluded hepatic veins. No other significant intraprocedural complication occurred. Fluoroscopy data was obtained for 206. All patients referred for TIPS underwent ultrasound-guided portal vein access irrespective of their BMI.
Table 1.
Primary indications for transjugular intrahepatic portosystemic shunt formation
| Indication | Number of patients (%) |
|---|---|
| Variceal hemorrhage | 95 (45) |
| Refractory ascites | 77 (36) |
| PV/SMV thrombosis | 17 (8) |
| Budd-Chiari syndrome | 12 (6) |
| Hepatic hydrothorax | 6 (3) |
| Not recorded | 4 (2) |
PV, portal vein; SMV, superior mesenteric vein.
Procedure
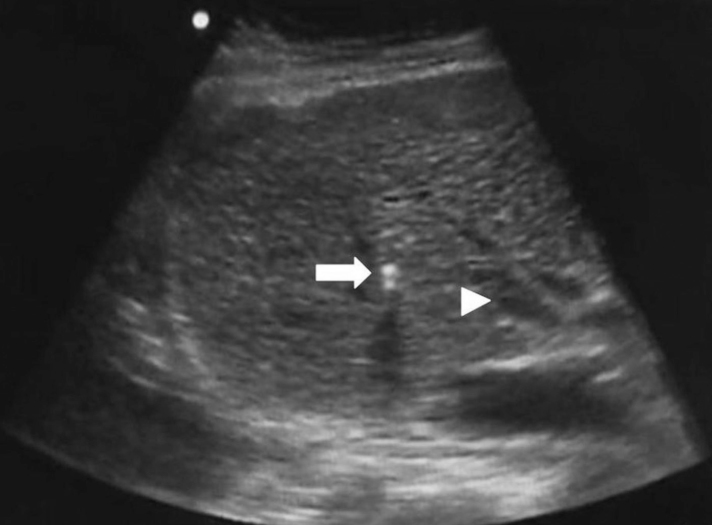
All procedures were performed under general anesthesia by five faculty level radiologists as the primary operator. All faculty level radiologists had more than ten years experience in interventional radiology. A 10 F sheath was inserted into the jugular vein (right, unless occluded) and the hepatic vein (usually right, although middle was also used) was cannulated using a 5 F Cobra catheter. A Rösch-Uchida transjugular liver access set (Cook Medical) was then introduced. At this stage the sonographer (radiology registrar or consultant) assumed their position adjacent to the patient’s right side, with the screen of the ultrasound machine (MicroMaxx, SonoSite or Sparq, Philips Healthcare) placed obliquely in relation to the patient such that both the operator and sonographer were able to see it clearly. A 3–5 MHz curvilinear abdominal probe was used. An oblique intercostal approach was used for insonation, usually between the 9th and 11th ribs depending on liver size, with small movements allowing for change of target from right hepatic vein to right portal vein. The Rösch-Uchida needle was extended via the sheath through the hepatic vein wall and directly visualized as it passed through the liver parenchyma, towards the right portal vein (Fig.). Color Doppler was used as required but B-mode usually sufficed for navigation. The operator could agitate the needle tip to aid visualization by ultrasound. The sonographer would provide verbal feedback relating to which direction the probe was moving to visualize the target vessels. The operator was then able to alter the degree of torque on the access set or position within the hepatic vein to enable PV puncture. If required the degree of angulation of the access set was altered to achieve PV access. Care was taken not to introduce air during access set or needle exchange as this significantly degrades the ultrasound image. Once access had been obtained into the right PV under direct ultrasound visualization, blood was aspirated via the catheter and direct portography was performed. Once a suitable position was confirmed, the TIPS technique progressed as previously described (25) with balloon dilatation of the track and deployment of VIATORR stent(s) (W.L. Gore & Associates).
Figure.
Intraprocedural ultrasound image showing needle tip (arrow) heading towards the target of the right portal vein (arrowhead).
Fluoroscopy
An Artis Zee fluoroscopy system (Siemens) with large flat panel detector was used for fluoroscopy, pulsed at 3 frames per second. Active coning was used at all times by the radiographer to minimize the field of view. Limited digital subtraction angiography (DSA) runs were performed as required, which ranged from a total time of five to 93 seconds (2 frames per second) with a mean of 35 seconds.
Results
In our retrospective study of 206 TIPS procedures, the mean fluoroscopy time was 12 min 9 s (SD, 14 min 38 s; 75th percentile, 26 min 8 s) and median fluoroscopy time was 16 min 17 s. Mean DAP was 40.3 Gy·cm2 (SD, 73.1 Gy·cm2; 75th percentile, 75.2 Gy·cm2) and median DAP was 38 Gy·cm2. Mean skin dose was 404 mGy (SD, 465 mGy; 75th percentile, 488.4 mGy) and median skin dose was 257 mGy.
Fluoroscopy times and doses were lower than RAD-IR DRLs as shown in Table 2. Median values for fluoroscopy time, DAP, and skin dose are shown compared with median values from the RAD-IR data in Table 2.
Table 2.
Fluoroscopy times, dose area products and skin doses for transjugular intrahepatic portosystemic shunts procedures in our cohort compared with reference levels from (22)
| Range | Mean±SD | 75th percentile | Median | ||
|---|---|---|---|---|---|
| Fluoroscopy time (min:s) | Data set | 04:48–112:40 | 12:09±14:38 | 26:08 | 16:17 |
|
| |||||
| RAD-IR DRL | 60:00 | 31:00 | |||
|
| |||||
| DAP (Gy·cm2) | Data set | 5.1–479.6 | 40.3±73.1 | 75.2 | 38 |
| RAD-IR DRL | 525 | 252 | |||
|
| |||||
| Skin dose (mGy) | Data set | 37–3257 | 404±465 | 488.4 | 257 |
| RAD-IR DRL | 3000 | 1489 | |||
SD, standard deviation; RAD-IR, Radiation Doses in Interventional Radiology Procedures study; DRL, dose reference level; DAP, dose area product.
Discussion
Our results show that transabdominal ultrasound-guided PV puncture during TIPS leads to shorter fluoroscopy times and lower patient dose, when compared with the published reference levels. Alongside the potential radiation dose benefits, direct visualization of the needle tip reduces likelihood of complications during PV puncture (6) and also allows a puncture site that will provide a good angle for the TIPS tract to be selected (9). No significant procedure-related complications occurred.
Alongside the “clockface” or “blind” fluoroscopic approach, wedged venography with both carbon dioxide and contrast has emerged as the favored technique in many centers (4). This is the technique we used prior to the introduction of transabdominal ultrasound. Although good images of the portal vein are produced, it has a number of limitations: only a two-dimensional image is generated and lateral imaging may be required, the anatomy becomes distorted when a large sheath is inserted, and DSA runs are required (i.e., higher dose). Additionally, complications can occur from this technique such as hepatic laceration and subcapsular hemorrhage (5, 9). Arterial portography is another method of navigation, which requires intra-arterial injection of contrast, although visualization of the PV can be suboptimal, particularly if there is hepatofugal PV flow (26). With the aforementioned methods, multiple needle passes are often required, increasing the length of fluoroscopy and the likelihood of inadvertent iatrogenic injury to local biliary, arterial, or capsular structures (2). Percutaneous methods of accessing or guiding the PV puncture have also been described (10), although these are not without risk as patients requiring TIPS are often coagulopathic.
More recent reports have outlined successful use of other techniques such as MRI guidance, which necessitates an open MRI scanner with co-located hybrid fluoroscopy (11) and use of intravascular ultrasound (mainly described in direct portocaval shunts rather than from the hepatic vein) (12). These methods require expensive equipment. In contrast, our approach does not require any additional investment as ultrasound capabilities are usually integrated in an angiography suite.
Entrance skin dose and effective dose are calculated indirectly utilizing surrogate measures to calculate dose, as with most commercial fluoroscopy systems, including our study. Although duration of fluoroscopy is the easiest dose parameter to measure, it is of little value when attempting to infer dose, as huge variations in patient and operator dose can occur due in complex procedures (27). DAP corresponds to the integral of air kerma across the entire x-ray beam emitted from the x-ray tube (28) and can act as a surrogate marker for dose to the patient’s skin (22, 29). Skin dose is an approximation of the total radiation dose to the skin. As the reference point from which this value is derived is not directly on the skin, this may tend to overestimate the probability of skin effects, given that the source moves in relation to the skin (30). Notably, neither DAP nor skin dose account for backscatter from the patient, which can generate substantial variation from the actual skin dose.
There is an increasing drive to reduce radiation doses, both from within the specialty and from healthcare and governmental bodies (31), with recent United States legislation aiming to link a proportion of reimbursement to the use of modern low dose equipment (32). This is particularly relevant as patients are being irradiated more frequently as the volume and indication for ionizing radiation-based imaging and interventions increases. Furthermore, as TIPS has shown its value in a number of conditions outside the initial indication of refractory variceal hemorrhage, more procedures are being undertaken. These tend to be concentrated in specialized centers, meaning those operators working there are more frequently performing them. Thus, increased scrutiny needs to be placed on the effective doses delivered to radiologists and methods to minimize this where possible (33).
We aim to follow the guidelines for dose reduction outlined by Miller et al. (30, 34) and use: collimation, optimized patient position in relation to the tube and detector, pulsed rather than continuous fluoroscopy with as low frame rate as possible, as few fluorographic DSA runs as possible, and image hold techniques where possible.
SIR-CIRSE guidelines suggest that patients should be followed up if dose parameters for any interventional radiology procedure exclude certain limits (fluoroscopy time >60 min, DAP 50 Gy·cm2 and cumulative dose 5000 mGy), advocating asking patients to self-examine the area irradiated two weeks postprocedure for evidence of skin changes and to seek medical advice. Notably these levels are similar to two of the DRLs outlined in RAD-IR. We feel this is prudent as TIPS remains a complex, challenging procedure and high doses do occur despite recent advances.
Our method of ultrasound guidance requires an additional radiologist to be present during the procedure, which has implications for departmental rostering. However, this is only necessary during the PV puncture from the hepatic vein, which in our center takes a mean of 27 min and median of 12 min (unpublished data). In addition this technique allows trainee interventional radiologists to learn the TIPS procedure.
There is a learning curve for the sonographer and anecdotally it is easier to perform this role when one has experience of performing TIPS oneself. Nonetheless we have had numerous situations where the sonographer was a junior radiologist and the procedure has been performed uneventfully. Other groups have described use of the ultrasound-guided portosystemic shunts: Livingstone outlined 19 cases (mainly caval-PV shunts) where the mean DAP was 63.9 Gy·cm2 and mean fluoroscopy time 19 min 12 s (16). More recently, Miraglia et al. (17) compared radiation dose from ultrasound-guided TIPS using flat panel detectors and image intensifier systems to fluoroscopic needle guidance for the PV puncture: DAP and fluoroscopy times were lower for ultrasound-guidance versus fluoroscopy. Notably the mean DAP of the US-guidance with flat panel system (i.e., similar to our system) was 129 Gy·cm2, three times higher than our series. Similarly, more fluoroscopy time was required (mean over 19 min); however, this maybe due to the difference between the flat panel system used, whereby we used a lower pulse rate of 3 frames per second compared with 7.5 frames per second in the series reported by Miraglia et al. (17).
In one of our cases, ultrasound-guided navigation was impossible since the liver was very fatty and therefore highly echogenic. Despite the majority of our patients being cirrhotic, often with shrunken irregular livers, there were few problems obtaining satisfactory images of the required anatomy (hepatic vein and right PV), and no problems for real-time guidance of the needle tip through the liver parenchyma. This included cases with portal vein thrombosis. However, the increasing prevalence of obesity and nonalcoholic fatty liver disease may impact the utility of ultrasound. It is pertinent to note that we used the Rösch-Uchida set whereas the only large cohort of TIPS performed with real-time ultrasound guidance for PV targeting was performed with the Colapinto needle (17). Therefore, real-time ultrasound guidance is feasible with both systems currently available for TIPS creation.
We have shown that our fluoroscopy time is almost one-third that of the RAD-IR study (23). The dose reduction with our technique is more significant: our mean DAP measurements is 14% of the RAD-IR study and skin dose is 16%. That this is proportionally a greater reduction than the fluoroscopy time is in part due to newer equipment and factors such as care positioning and pulsed as opposed to continuous fluoroscopy. The RAD-IR study was performed in 1999–2002 across a number of centers and it is notable how fluoroscopy technology and techniques have improved significantly over the last 15 years. In addition, older wedged venography techniques required more DSA, as well as a greater number of oblique and lateral projections, which increased dose.
Although the RAD-IR data are the only published DRLs for TIPS, there are some limitations with them. As the data is from over 10 years ago, there have been significant improvements in fluoroscopy equipment in the interim. All data were obtained from academic teaching hospitals which could potentially skew the dosimetry data in either direction: trainees performing procedures tend to take longer and require more fluoroscopy due to inexperience and unfamiliarity (35), and these centers tend to be regional referral centers and see more complex cases; conversely, the operators in these centers tend to be more experienced and may require less fluoroscopy. Notably our institution is also a tertiary referral center so assumptions in this regard are equally applicable to our results. Finally the dose data were not adjusted for body habitus, but rather a standardized weight, which may introduce inaccuracy due to the increasingly prevalence of obesity, which can increase doses substantially (30).
In conclusion, we have shown that transabdominal ultrasound-guidance of PV puncture during TIPS generates substantially lower doses than published DRLs.
Main points.
Using transabdominal ultrasound to guide during transjugular intrahepatic portosystemic shunt (TIPS) formation results in less fluoroscopy time than published dose reference levels (DRLs).
Using transabdominal ultrasound to guide TIPS formation leads to lower radiation doses to the patient than published DRLs.
These reductions in dose may have beneficial effects in reducing patient and operator dose in what is one of the most complex and high dose interventional radiology procedures.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rössle M, Deibert P, Haag K, et al. Randomised trial of transjugular-intrahepatic-portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet. 1969;349:1043–1049. doi: 10.1016/s0140-6736(96)08189-5. https://doi.org/10.1016/S0140-6736(96)08189-5. [DOI] [PubMed] [Google Scholar]
- 2.Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK. The transjugular intrahepatic portosystemic shunt: an update. Am J Roentgenol. 2012;199:746–755. doi: 10.2214/AJR.12.9101. https://doi.org/10.2214/AJR.12.9101. [DOI] [PubMed] [Google Scholar]
- 3.Han G, Qi X, He C, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 2011;54:78–88. doi: 10.1016/j.jhep.2010.06.029. https://doi.org/10.1016/j.jhep.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Owen AR, Stanley AJ, Vijayananthan A, Moss JG. The transjugular intrahepatic portosystemic shunt (TIPS) Clin Radiol. 2009;64:664–674. doi: 10.1016/j.crad.2008.09.017. https://doi.org/10.1016/j.crad.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Semba CP, Saperstein L, Nyman U, Dake MD. Hepatic laceration from wedged venography performed before transjugular intrahepatic portosystemic shunt placement. J Vasc Interv Radiol. 1996;7:143–146. doi: 10.1016/s1051-0443(96)70751-0. https://doi.org/10.1016/S1051-0443(96)70751-0. [DOI] [PubMed] [Google Scholar]
- 6.Davis AG, Haskal ZJ. Extrahepatic portal vein puncture and intra-abdominal hemorrhage during transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol. 1996;7:863–866. doi: 10.1016/s1051-0443(96)70863-1. https://doi.org/10.1016/S1051-0443(96)70863-1. [DOI] [PubMed] [Google Scholar]
- 7.Richter GM, Noeldge G, Palmaz JC, et al. Transjugular intrahepatic portacaval stent shunt: preliminary clinical results. Radiology. 1990;174:1027–1030. doi: 10.1148/radiology.174.3.174-3-1027. https://doi.org/10.1148/radiology.174.3.174-3-1027. [DOI] [PubMed] [Google Scholar]
- 8.Uflacker R, Reichert P, D’Albuquerque LC, de Oliveira e Silva A. Liver anatomy applied to the placement of transjugular intrahepatic portosystemic shunts. Radiology. 1994;191:705–712. doi: 10.1148/radiology.191.3.8184050. https://doi.org/10.1148/radiology.191.3.8184050. [DOI] [PubMed] [Google Scholar]
- 9.Kirby JM, Cho KJ, Midia M. Image-guided intervention in management of complications of portal hypertension: more than TIPS for success. Radiographics. 2013;33:1473–1496. doi: 10.1148/rg.335125166. https://doi.org/10.1148/rg.335125166. [DOI] [PubMed] [Google Scholar]
- 10.Haskal ZJ, Duszak R, Furth EE. Transjugular intrahepatic transcaval porto-systemic shunt: the gun-sight approach. J Vasc Interv Radiol. 1996;7:139–142. doi: 10.1016/s1051-0443(96)70750-9. https://doi.org/10.1016/S1051-0443(96)70750-9. [DOI] [PubMed] [Google Scholar]
- 11.Stephen T, Kee AG. MR-guided transjugular intrahepatic portosystemic shunt creation with use of a hybrid radiography/MR system. J Vasc Interv Radiol JVIR. 2005;16:227–234. doi: 10.1097/01.RVI.0000143766.08029.6E. https://doi.org/10.1097/01.RVI.0000143766.08029.6E. [DOI] [PubMed] [Google Scholar]
- 12.Luo X, Ye L, Zhou X, Tsauo J, Zhou B, Zhang H, Zhang X, Li X. C-Arm cone-beam volume CT in transjugular intrahepatic portosystemic shunt: initial clinical experience. Cardiovasc Intervent Radiol. 2015;38:1627–1631. doi: 10.1007/s00270-015-1087-2. https://doi.org/10.1007/s00270-015-1087-2. [DOI] [PubMed] [Google Scholar]
- 13.Petersen B, Binkert C. Intravascular ultrasound–guided direct intrahepatic portacaval shunt: midterm follow-up. J Vasc Interv Radiol. 2004;15:927–938. doi: 10.1097/01.RVI.0000133703.35041.42. https://doi.org/10.1097/01.RVI.0000133703.35041.42. [DOI] [PubMed] [Google Scholar]
- 14.Hidajat N, Kreuschner M, Röttgen R, Schröder R-J, Schmidt S, Felix R. Placement of transjugular intrahepatic portosystemic shunt via the left hepatic vein under sonographic guidance in a patient with right hemihepatectomy. Acta Radiol. 2003;44:363–365. doi: 10.1080/j.1600-0455.2003.00097.x. https://doi.org/10.1080/j.1600-0455.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 15.Longo JM, Bilbao JI, Rousseau HP, et al. Color Doppler-US guidance in transjugular placement of intrahepatic portosystemic shunts. Radiology. 1992;184:281–284. doi: 10.1148/radiology.184.1.1609093. https://doi.org/10.1148/radiology.184.1.1609093. [DOI] [PubMed] [Google Scholar]
- 16.Livingstone RS, Keshava SN. Technical note: Reduction of radiation dose using ultrasound guidance during transjugular intrahepatic portosystemic shunt procedure. Indian J Radiol Imaging. 2011;21:13–14. doi: 10.4103/0971-3026.76046. https://doi.org/10.4103/0971-3026.76046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miraglia R, Maruzzelli L, Cortis K, et al. Radiation exposure in transjugular intrahepatic portosystemic shunt creation. Cardiovasc Intervent Radiol. 2016;39:210–216. doi: 10.1007/s00270-015-1164-6. https://doi.org/10.1007/s00270-015-1164-6. [DOI] [PubMed] [Google Scholar]
- 18.Koenig TR, Wolff D, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. Am J Roentgenol. 2001;177:3–11. doi: 10.2214/ajr.177.1.1770003. https://doi.org/10.2214/ajr.177.1.1770003. [DOI] [PubMed] [Google Scholar]
- 19.George T, Nahass LC. Fluoroscopy-induced radiodermatitis after transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 1998;93:1546–1549. doi: 10.1111/j.1572-0241.1998.00479.x. https://doi.org/10.1111/j.1572-0241.1998.00479.x. [DOI] [PubMed] [Google Scholar]
- 20.Cante V, Doutre MS. Radiodermite chronique de la région thoracique antérieure après dérivation intrahépatique porto-systémique par voie transjugulaire. Ann Dermatol Vénéréologie. 2011;138:424–425. doi: 10.1016/j.annder.2011.01.041. https://doi.org/10.1016/j.annder.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Hidajat N, Wust P, Kreuschner M, Felix R, Schröder RJ. Radiation risks for the radiologist performing transjugular intrahepatic portosystemic shunt (TIPS) Br J Radiol. 2006;79:483–486. doi: 10.1259/bjr/67632946. https://doi.org/10.1259/bjr/67632946. [DOI] [PubMed] [Google Scholar]
- 22.Miller DL, Balter S, Cole PE, et al. Radiation doses in interventional radiology procedures: The RAD-IR study part II: skin dose. J Vasc Interv Radiol. 2003;14:977–990. doi: 10.1097/01.rvi.0000084601.43811.cb. https://doi.org/10.1097/01.RVI.0000084601.43811.CB. [DOI] [PubMed] [Google Scholar]
- 23.Miller DL, Kwon D, Bonavia GH. Reference Levels for Patient Radiation Doses in Interventional Radiology: Proposed Initial Values for U.S. Practice. Radiology. 2009;253:753–764. doi: 10.1148/radiol.2533090354. https://doi.org/10.1148/radiol.2533090354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aroua A, Rickli H, Stauffer JC, et al. How to set up and apply reference levels in fluoroscopy at a national level. Eur Radiol. 2006;17:1621–1633. doi: 10.1007/s00330-006-0463-3. https://doi.org/10.1007/s00330-006-0463-3. [DOI] [PubMed] [Google Scholar]
- 25.Owen AR, Stanley AJ, Vijayananthan A, Moss JG. The transjugular intrahepatic portosystemic shunt (TIPS) Clin Radiol. 2009;64:664–674. doi: 10.1016/j.crad.2008.09.017. https://doi.org/10.1016/j.crad.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Wachsberg RH, Bahramipour P, Sofocleous CT, Barone A. Hepatofugal flow in the portal venous system: pathophysiology, imaging findings, and diagnostic pitfalls. Radiographics. 2002;22:123–140. doi: 10.1148/radiographics.22.1.g02ja20123. https://doi.org/10.1148/radiographics.22.1.g02ja20123. [DOI] [PubMed] [Google Scholar]
- 27.Balter S. Capturing patient doses from fluoroscopically based diagnostic and interventional systems. Health Phys. 2008;95 doi: 10.1097/01.HP.0000327650.37315.2f. https://doi.org/10.1097/01.hp.0000327650.37315.2f. [DOI] [PubMed] [Google Scholar]
- 28.Stecker MS, Balter S, Towbin RB, et al. Guidelines for patient radiation dose management. J Vasc Interv Radiol. 2009;20:S263–273. doi: 10.1016/j.jvir.2009.04.037. https://doi.org/10.1016/j.jvir.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher DW, Miller DL, Balter S, Taylor MA. Comparison of four techniques to estimate radiation dose to skin during angiographic and interventional radiology procedures. J Vasc Interv Radiol. 2002;13:391–397. doi: 10.1016/s1051-0443(07)61742-4. https://doi.org/10.1016/S1051-0443(07)61742-4. [DOI] [PubMed] [Google Scholar]
- 30.Miller DL, Balter S, Schueler BA, Wagner LK, Strauss KJ, Vañó E. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321–332. doi: 10.1148/radiol.10091269. https://doi.org/10.1148/radiol.10091269. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration. Initiative to reduce unnecessary radiation exposure from medical imaging - white paper: initiative to reduce unnecessary radiation exposure from medical imaging. Available at: http://www.fda.gov/Radiation-EmittingProducts/RadiationSafety/RadiationDoseReduction/ucm199994.htm.
- 32.MITA lauds CMS mandate on medical imaging. AuntMinnie.com; [Accessed March 28, 2015]. Available at: http://www.auntminnie.com/index.aspx?sec=nws&sub=rad&pag=dis&itemid=107874. [Google Scholar]
- 33.Miller DL, Vañó E, Bartal G, et al. Occupational radiation protection in interventional radiology: a joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc Intervent Radiol. 2010;33:230–239. doi: 10.1007/s00270-009-9756-7. https://doi.org/10.1007/s00270-009-9756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller DL, Balter S, Noonan PT, Georgia JD. Minimizing radiation-induced skin injury in interventional radiology procedures. Radiology. 2002;225:329–336. doi: 10.1148/radiol.2252011414. https://doi.org/10.1148/radiol.2252011414. [DOI] [PubMed] [Google Scholar]
- 35.Stuart S, Mayo JR, Ling A, et al. Retrospective study of the impact of fellowship training on two quality and safety measures in uterine artery embolization. J Am Coll Radiol. 2014;11:471–476. doi: 10.1016/j.jacr.2013.09.020. https://doi.org/10.1016/j.jacr.2013.09.020. [DOI] [PubMed] [Google Scholar]