ABSTRACT
MicroRNAs are short non-protein coding RNA molecules involved in the epigenetic regulation of gene expression. Recently, extracellular microRNAs have been described in body fluids that might enable epigenetic communication between distant tissues. Being highly conserved molecules, exogenous xeno-microRNAs from different species could affect gene expression in the host even in a cross-kingdom fashion. Several data underline the relevance of microRNA-mediated communication between virus and host, and there are some experimental data showing that plant- or animal-derived dietary microRNAs might have gene expression modulating activity in humans. Milk-derived microRNAs might be involved in the “epigenetic priming” of the baby. Exogenous microRNAs might be hypothesized to be implicated in disease pathogenesis, e.g. in tumors. Major questions remain to be addressed including the amount of xeno-microRNAs needed for biological action or routes for microRNA delivery. In this brief review, experimental data and hypotheses on the potential pathogenic inter-species relevance of microRNA are presented.
KEYWORDS: Body fluid, diet, inter-individual, inter-species, microRNA, milk, xeno-microRNA
Abbreviations
- AGO2
Argonaute-2 protein
- dsRNA
double stranded RNA
- FoxO1
forkhead box class O1A
- FOXP3
forkhead box P3
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- LDLRAP1
low-density lipoprotein receptor adapter protein 1
- miRNA, miR
microRNA
- mTORC1
mechanistic target of rapamycin complex 1
- pre-miRNA
precursor microRNA
- pri-miRNA
primary microRNA
- RISC
RNA-induced silencing complex
- RUNX2
runt related transcription factor 2
- siRNA
small interfering RNA
- TCF7
transcription factor 7
- TLR
Toll-like receptor
- Treg
regulatory T-cell
- ZEB1
zinc finger E-box binding homeobox 1
Introduction
MicroRNAs (miRNA, miR) are short non-protein coding RNA molecules that are involved both in the nuclear and the post-transcriptional regulation of gene expression. miRNAs are the endogenous mediators of RNA-interference forming part of the epigenetic machinery.1 In this regard, the concept epigenetic means post-transcriptional influence on gene expression without affecting the DNA sequence.2,3 miRNAs are predicted to regulate 30-60 % of all genes in humans.3 Their genes are located mostly in non-coding genomic regions (often termed the dark matter of the genome), or less frequently in exonic or intronic regions of protein-coding genes.4 Most miRNA genes are transcribed to primary miRNAs (pri-miRNA) by RNA polymerase II.5 miRNAs undergo a complex maturation process comprising the most typical and best described canonical pathway and other alternative routes. In the canonical pathway, pri-miRNAs are cleaved by the so-called microprocessor complex in the nucleus. The complex includes Drosha and DGCR8 (Di-George syndrome critical/chromosomal region 8).6 The nascent precursor miRNA (pre-miRNA) is delivered by Exportin-5 and Ran GTP-ase proteins from the nucleus to the cytoplasm.7 The mature single-stranded, 19-25 nucleotide long miRNA is generated by the endoribonuclease Dicer. RISC (RNA-induced silencing complex) is composed of the mature miRNA, the transactivation-responsive RNA-binding protein (TRBP) and Argonaute-2 protein (AGO2).8
miRNAs are highly conserved molecules showing high degrees of complementarity even between evolutionarily distant species such as worms, insects and mammals.9 miRNAs arose independently in plants, but their basic mechanism of action in post-transcriptional regulation is similar in all eukaryotes.9,10 miRNAs are also very stable that is exemplified by the fact that miRNAs can be effectively retrieved and analyzed from archived tissue samples.11
The most typical and best-described action of miRNAs is the post-transcriptional modulation of gene expression, whereby the mature single-stranded miRNA in RISC binds the 3′ untranslated region (3′ UTR) of its target mRNA (mRNA). Depending on the degree of complementarity between the miRNA and target mRNA sequences, translational inhibition or mRNA degradation follows. Partial complementarity resulting in translational inhibition is characteristic for animals, whereas perfect complementarity and subsequent mRNA degradation is typical for plants.12 On the other hand, there are also findings showing that miRNAs can have positive effects on gene expression, as well, further expanding the biological relevance of miRNAs.13
Major features of miRNAs are related to their pleiotropic and synergistic actions, whereby a single miRNA can have several potential mRNA targets, moreover, a mRNA usually also has multiple miRNA binding sites. miRNAs binding to a single mRNA often act in a synergistic fashion. The expression of miRNAs is tissue specific, i.e. the miRNA expression patterns between different tissues are quite different. Furthermore, the target pattern of a given miRNA and thus its action on gene expression is also tissue specific, as miRNA targets can be very different in different tissues.14 The outstanding biological relevance of miRNAs is confirmed among others in the regulation of cell proliferation, differentiation, apoptosis, immune response, as well as in numerous pathological conditions such as tumorigenesis and infections.15 In tumors, miRNAs are classified as oncogenes and tumor suppressors following the classical oncogene-tumor suppressor dichotomy.16
Novel data have revealed that beside their tissue counterparts, miRNAs are found and stable in different body fluids, as well.17 According to our current knowledge, miRNA release from the cell can occur through 3 major pathways: i. passive outflow due to cellular damage (e.g., inflammation or necrosis), and active secretion in ii. extracellular vesicles (apoptotic bodies, exosomes and microvesicles), or iii. in macromolecular complexes with AGO proteins (mostly AGO2) and high density lipoprotein (HDL).18 Extracellular miRNAs in membrane vesicles and macromolecular complexes are very stable in the blood.19 The miRNA content of exosomes has been shown to be quite different from that of secreting cells suggesting selective packaging.20,21,22,23 Unfortunately, the molecular mechanism for selective packaging is still unclear.
Experimental data support that miRNAs in membrane-bound vesicles24 and complexed with HDL25 might enter cells and influence gene expression. Several findings support that exosomal miRNAs secreted from tumors are involved in intratumoral epigenetic communication by influencing nearby tumor cells, but non-tumorous cells (e.g., interstitial, immune or endothelial cells) are also modulated. These interactions might be implicated in tumor invasion, metastasis formation etc.15,26,27,28 Considering their potential to modulate gene expression even in distant cells and tissues, circulating miRNA might be regarded as hormones conveying epigenetic information.29 This is, however, only a hypothesis at present, whose biological relevance is rather difficult to test.29,30
In contrast with this paradigm of cell-to-cell communication,23 a conflicting hypothesis (“trash theory” or “hypothesis of cellular byproducts”) argues that AGO-associated extracellular miRNAs should be regarded as molecular debris.23,31 Moreover, it is debated whether miRNAs are really included in membrane vesicles, as this might also be related to analytic problems.23 In very recent studies, most exosomes have turned out to lack miRNA,23,32,33 and the miRNA copy number in individual exosomes appears to be very low.32 These findings argue against the biological activity of extracellular miRNA. From a diagnostic point of view, however, even if only representing cellular debris, extracellular miRNAs are relevant as they can be exploited as minimally invasive biomarkers in different pathological conditions.34
As often observed with conflicting theories, the truth probably lies in between the two. Recent findings have shown that AGO-bound miRNAs might cross gap junctions and modulate gene expression in recipient cells.35 The low miRNA content of individual exosomes does not exclude their biological activity either, and exosomes that are relatively rich in miRNAs might also exist.23,36
A further mechanism for the biological activity of extracellular miRNA via Toll-like receptors (TLR) has been recently reported: tumor-cell secreted microRNAs have been found to bind TLRs in immune cells and to induce a prometastatic inflammatory response facilitating tumor growth.37
Based on these findings, we can conclude that the biological relevance of extracellular miRNAs is controversial at present, but extracellular miRNA cannot be regarded as fully inert and they might have some biological activity. This is a very intriguing hypothesis.
Given their stability and presence in body fluids, in addition to their potential intraindividual actions, miRNAs can be hypothesized to be involved in inter-individual epigenetic communication, whereby miRNAs released by an individual could have actions in others having contact with their body fluids. Certainly, effective miRNA transfer and uptake mechanisms should be present to ensure their biological activity in another organism. An even more intriguing hypothesis is related to their conserved structure and way of action that might form basis for a potential inter-species miRNA activity that could even exist in a cross-kingdom fashion mostly via the ingestion of dietary miRNAs. miRNAs derived from other species are termed xeno-miRNAs. In the following, we will present experimental data and hypotheses on the potential inter-individual and inter-species activity of miRNAs in an attempt to highlight this fascinating, but still controversial field of contemporary science. Several data underline the relevance of miRNA-mediated communication between virus and host, but this topic will be covered in detail in another article of this issue.
miRNAs in interindividual communication
Dietary milk-derived miRNAs
Among different body fluids, breast milk contains the highest amount of miRNA.17,38 Milk miRNAs are secreted in exosomes and they are extremely stable under different conditions such as very low and high pH, repeated cycles of freezing and thawing or even extended storage.39,40 The largest amount of milk miRNAs is derived from the lactating mammary epithelium.41
If miRNAs in the milk can be absorbed, these could have epigenetic regulatory roles in the offspring, i.e., miRNAs might be involved in the “epigenetic priming” of the baby (so-called functional hypothesis).42,43 The functional activity of milk-derived miRNAs is not universally accepted, and the other, nutritional hypothesis argues that miRNAs are not absorbed into the circulation, but they are degraded to nucleotides, which merely serve as nutrients.42 The nutritional hypothesis is based on 3 mouse models42 (i. transgenic mice overexpressing miR-30b,44 and ii. miR-375 and iii. miR-200c/141 knockout mice45). These models have not revealed major associations between changes in milk miRNA concentrations and in tissues or plasma of the offspring, however, all these 3 models have limitations.42 In the first model, transgenic mice overexpressing miRNA-30b have been investigated. miR-30b is involved in the regulation in lactation,44 and it inhibits phagocytosis in myeloid inflammatory cells, as well.42,46 Significantly increased concentration of miR-30b was observed in the milk of transgenic mice, but no alteration in its concentration in milk-fed pup tissues was found.47 A major weakness of this study is that the authors have not evaluated miR-30b concentration in exosomes, and overexpressed miR-30b might interfere with the formation of exosomes due to defects in mammary epithelium of transgenic mice.42 In the other 2 models, miR-375 and miR-200c/141 knockout mice fed by wild-type wet nurse mice were explored.42,45 Only a modest increment in the plasma concentration of these 2 miRNAs was noted, and the authors concluded that milk miRNAs serve just as a dietetic source. However, both miR-375 and miR-200c might also affect exosome formation via exocytotic/endocytotic pathways,42,48,49 and the required amount of miRNAs for biological activity is also intensively debated.32,50 It would be necessary to develop models that are more physiological, e.g. by studying the transfer of labeled milk miRNAs in healthy animals.42
There are epidemiological findings showing that breastfeeding is a preventing factor against allergy,52 and milk-derived miRNAs might be relevant in this phenomenon. miR-155, which does not belong to the most prevalent miRNAs in milk,17 but plays an essential role in the development of immune system might exert regulatory functions on thymus-derived T-cells. Furthermore, there are hypotheses that milk-derived miR-155 could prevent allergy by increasing forkhead box P3 (FOXP3) expression and consequently the efficiency of regulatory T-cells (Treg).53,54 It is well-known that decreased Treg functioning is accompanied by the increased prevalence of T helper 2 cells which are crucial in the emergence of atopy.55 (Fig. 1)
Figure 1.
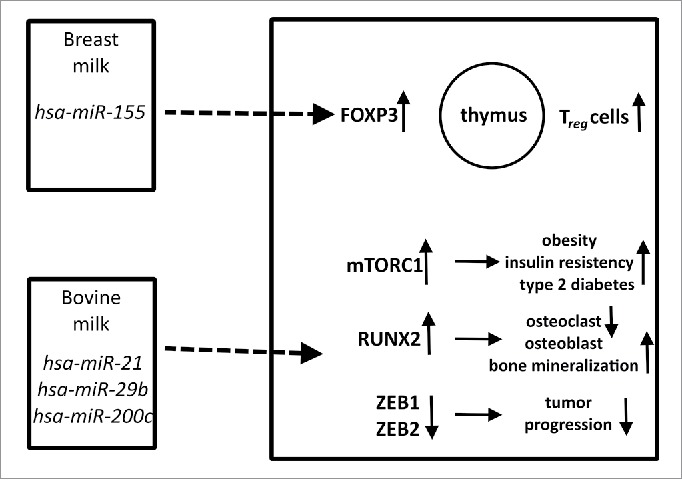
Biological relevance of miRNAs in human and bovine milk affecting various pathways. miR-155 in breast milk might affect thymic T-cell maturation, whereas miRNAs in bovine milk could be relevant in obesity, diabetes mellitus, bone metabolism and maybe in tumor progression. Upward and downward arrows represent increase or decrease, respectively.
According to the functional hypothesis, milk is not only important as a source of nutrients and immunoglobulins, but also miRNAs from milk exosomes could serve as epigenetic modulators.56 It can be hypothesized that similar to the transfer of immunoglobulins, miRNA might be more effectively absorbed in babies than in adults.
Other potential routes of inter-individual miRNA-mediated communication
A further potential inter-individual way of miRNA transfer could be represented by blood transfusion,57 but there are no data at present showing that transferred miRNA would have pathogenic relevance. The biological relevance of miRNAs in other body fluids as inter-individual mediators is fairly hypothetical, as there are no experimental findings to support these either. Sperm- and saliva-derived miRNAs might easily affect other individuals in close relationships.30 It can even be hypothesized that miRNAs derived from urine and stool might influence gene expression in others, but in areas with developed sewage systems the concentration of these miRNAs would be so low that their biological effects are hardly conceivable.30
Dietary xeno-miRNA in inter-species communication
Beside the potential intraspecies inter-individual activity of milk-derived miRNAs, a further question could be raised regarding the relevance of animal-derived milk. Considering the high-degree of homology between animal and human miRNAs, milk-derived miRNAs could also be relevant in an inter-species epigenetic communication.
Recent findings suggest that bovine miRNAs are bioavailable for humans as a result of the absorption of milk exosomes to the human circulation.58 Not only human macrophages can annex bovine milk exosomes59 but vascular endothelial cells, too.60 Furthermore, milk exosomes appear even in peripheral tissues such as liver, spleen and lungs.60
miRNA concentrations significantly decrease during milk pasteurization and production.61 Similarly to human breast feeding, the consumption of raw bovine milk is also associated with decreased risk of allergy and asthma,62 and it cannot be excluded that bovine miRNAs are implicated in these phenomena. A recent study proved that bovine milk- derived miRNAs can be absorbed and appear in human blood after milk consumption,63 but this finding is also debated.64,65 The bovine milk-derived xeno-miRNA, miR-29b which is identical to its human counterpart,66 has been shown to raise the expression of the runt related transcription factor 2 (RUNX2) in human blood peripheral mononuclear cells.63 As RUNX2 is involved in the regulation of bone homeostasis by inducing osteoblasts and inhibiting the osteoclast differentiation in the host, the ingested bovine miR-29b xeno-miRNA might be hypothesized to induce bone mineralization.67,68 Milk consumption might thus promote bone mineralization not only as a classical source of dietary calcium, but even via its miRNA content.
Moreover bovine milk exosomal miR-21 and miR-29b could be relevant in obesity, insulin resistance and type 2 diabetes mellitus via the nutrient sensitive mechanistic target of rapamycin complex 1 (mTORC1)69,70 and other pathways such as the inhibition of forkhead box class O1A (FoxO1) transcription factor having a key role in β-cell homeostasis by miR-21.71 miR-29b might activate mTORC1 by increasing the amount of intracellular branched-chain amino acids via inhibiting the enzyme responsible for their catabolism.72 (Fig. 1)
Another bovine derived miRNA, miR-200c which is also identical to its human counterpart66 targets zinc finger E-box binding homeobox 1 and 2 (ZEB1 and ZEB2) proteins that are transcriptional repressors of E-cadherin,73 and thus acts as a tumor suppressor in different models.73,74 Bovine milk miR-200c has also been shown to be absorbed in humans, but no effect on ZEB1 expression has been noted.63 It has to be mentioned, however, that another study could not confirm these results on milk-derived miRNA despite working with the same samples.75
The hypothetical cancer-preventing activity of a bovine milk miRNA is intriguing, and there are some available data suggesting that milk consumption might reduce cancer risk,76,77 but the relevance of miRNA in these observations is only hypothetical at present.
Dietary xeno-miRNA acting in a cross-kingdom fashion
In a revolutionary report published in 2012, exogenous rice-derived miRNAs were claimed to be found in the human circulation and one of these plant-derived miRNAs (rice-derived miR-168a) modified gene expression in the host.78 It was earlier known that exogenous miRNA could be detected in the blood of healthy individuals,79,80 but this was the first report to show that a xeno-miRNA could affect gene expression in the recipient. As plant miRNAs have a different chemical structure compared to animal miRNAs (the terminal nucleotide is modified by 2′-O methylation in plant miRNAs),81 plant miRNAs can be clearly identified. Rice-derived miR-168a was shown to target the low-density lipoprotein receptor adapter protein 1 (LDLRAP1), which plays a crucial role in cholesterol metabolism in mammals.82 Functional in vitro and in vivo studies on mice demonstrated that the binding of miR-168a to LDLRAP1 mRNA causes inhibition of its expression, and this leads to decreasing low density lipoprotein (LDL) removal from plasma and consequently increased LDL serum concentration.78 Despite findings showing that down-regulation of LDLRAP1 raises LDL concentration,83 the current clinical experience has not provided any evidence that rice-based diet would result in increased serum LDL concentration and potentially higher cardiovascular morbidity.84,85
Following an initial upheaval implying that diet should be now regarded not only as a source of nutrients but also as a potential source for epigenetic, gene expression modulating miRNAs,86 several data appear to weaken this “dietary xeno-miRNA” hypothesis.87 First, the biological relevance was argued, suggesting that the presence of the exogenous miR-168a was just an artifact as a result of sequencing methods.88
On the other hand, the available data are controversial on the efficiency of gastrointestinal absorption for xeno-miRNAs. While the animal-derived miRNAs might be absorbed in significant quantity and might have biological relevance,63 the amount of absorbed plant-derived miRNAs did not reach meaningful quantity in some investigations.57 First, only minor expression changes have been observed in the plasma concentration of 3 plant miRNAs (miR-156a, miR-159a, miR-169a) in healthy athletes after oral administration.57 Moreover, when miR-21 knock-out mice were fed with miR-21 rich nutrition no significant alteration either in the plasma or in the tissues of the recipient could be observed.57
A further interesting observation claimed that plant-derived miRNAs can be detected in human and porcine milk exosomes.89 Moreover human targets of these miRNAs have been raised supposing a potential cross-kingdom regulatory effect in the host.89 However, a recent study debates these findings, claiming that the detected miRNAs are just contaminants.90 Nevertheless, if plant-derived miRNAs would be present in the milk, this would imply that the diet of the mother might affect gene expression in the offspring via the ingested xeno-miRNA.
The biological relevance of diet-derived miRNA is thus rather controversial,57 but given the available positive findings, the field warrants further investigations.87,91
Major questions to be addressed related miRNA acting among individuals or inter-species
Two major questions have to be addressed for establishing the biological relevance of miRNA in inter-individual or inter-species regulation of gene expression:
Required amount of miRNAs for biological activity
The concentration of individual miRNA in the circulation is in the femtomolar range.92 It is difficult to determine what is the minimal amount of miRNA that is sufficient for biological activity, and whether such an amount can be efficiently delivered to the host via e.g., the gastrointestinal tract. It has been suggested that miRNA copy number of about one hundred to one thousand per cell would be needed to affect gene expression.51,93,94 Regarding the nuclear regulation by miRNAs, it can be imaginable that even fewer copies per cell is enough.87 Recent findings showing that individual exosomes contain very low copy numbers of miRNA would argue against a major biological role for extracellular miRNAs,32 but the copy number of their targets and their affinity to mRNAs might also be relevant.23,50 For inhibiting the transformation of a single cell, much fewer miRNA would be necessary than for influencing a macroscopic tumor. It is difficult to predict whether the question for the required amount of miRNA can be answered in general at all, as the gene expression modulating activity of different miRNAs and their intra- and extracellular half-lives might be different, and even the presence of miRNA inhibitors cannot be excluded. Even the existence of a miRNA receptor has been proposed92 that would bypass the question of low individual miRNA concentrations, but there are no experimental findings to date supporting this hypothesis.
The mechanism and efficiency of xeno-miRNA transfer via the gastrointestinal barrier
The investigation of C. elegans provided clue findings for the gastrointestinal transfer of double stranded RNA (dsRNA) mediating RNA interference.95,96,97 The proteins Sid-1 and Sid-2 are major effectors in this phenomenon. Sid-1 is a conserved multipass transmembrane protein and has homologs even in mammals. It is supposed to operate as a dsRNA channel.97,98 Sid-2 is a single-pass transmembrane protein which is located at the luminal membrane of the intestine,99 and is also involved in the absorption of ingested ds RNA.100 According to the current working hypothesis, Sid-2 binds long dsRNA first for endocytosis then the dsRNA is presented to Sid-1. For an efficient uptake of dsRNA to the intestinal cell cytoplasm, the activation of Sid-1 by Sid-2 is inevitable.
The mechanism of RNA uptake in vertebrates including humans has not been clarified, yet.87 A potential effector could be the transmembrane protein Sidt-1 that is the human ortholog of sid-1.101 There are data that Sidt-1 is able to transfer small interfering RNA (siRNA) and miR-21.102,103 Moreover, as discussed previously, TLRs are able to recognize dsRNA104 and stimulation of specific TLRs via miRNAs have been described, as well.37
Furthermore, there are hypotheses suggesting that the transfer of RNA could take place via the uptake of extracellular vesicles containing RNAs, by transcytosis from the lumen to the cytoplasm or across transmembrane RNA channels.87
It is largely unknown, however, how efficient the gastrointestinal uptake of small molecular RNA molecules might be78,91 and further investigations are needed to answer this crucial question.
To complicate the complexity of this question even further, diseases which increase the permeability of the gastrointestinal tract (e.g. inflammatory bowel diseases, ischemic bowel lesions, even gastrointestinal tumors) might significantly affect the absorption of miRNAs, and dietary miRNAs could have more pronounced biological activity under these conditions.
Hypotheses relating xeno-miRNA and diseases
The major disease entity where xeno-miRNA might be of relevance could be tumors. We have recently presented 2 hypotheses on the potential regulatory role of circulating miRNA in tumor development that could also be extended to xeno-miRNA.
By studying the circulating miRNA expression profiles in healthy individuals, we have noted that miRNAs with predominant tumor suppressor activity appear to be overrepresented in the human blood.105 We hypothesized that this tumor suppressor potential of circulating miRNAs could be relevant in preventing tumor formation, by inhibiting the early stages of tumorigenesis. In contrast with cancer immunosurveillance that is a rather slow process, such miRNA-mediated tumor suppressing activity could be very rapid and thus might complement the immune activity for tumor prevention. This is certainly a hypothesis and major counter arguments could be deployed against it including the very low concentration of individual miRNA in the circulation (femtomolar range),92 the dual nature of miRNA (the same miRNA can be oncogenic in one, and tumor suppressor in another tissue), their tissue specificity etc.105
By extending this hypothesis, it cannot be excluded that absorbed exogenous miRNA might interfere with tumor formation, as well. Unilateral diets including some miRNA in relatively high quantity and excluding others might lead to the relative overrepresentation of some miRNA. If these have predominant tumor suppressor or oncogenic potential, such diet-induced mechanisms might be relevant for tumor formation that might have a role among the well-known diet-related tumorigenic factors. This hypothetic tumor-related activity of dietary xeno-miRNA would be most easily realized in gastrointestinal tumors, as the tumor-related breakdown of the gastrointestinal barrier might facilitate the access of miRNA.
A major support for the relevance of a cross-kingdom acting xeno-miRNA in human cancer has been published recently.106 This was the first study to report that a plant-derived miRNA might affect tumor formation in humans. An inverse correlation between the incidence and progression of breast cancer and the quantity of plant-derived miR-159 in human sera has been established. Moreover, a dose-dependent growth reduction of breast tumor xenografts has been observed after oral administration of a miR-159 mimic in mice. The biological background of this phenomenon might be related to a link between plant miR-159 and its validated human target transcription factor 7 (TCF7). TCF7 plays a crucial role in the progression of breast cancer107,108 via the up-regulation of the Wnt signaling pathway (Fig. 2A).109 Inhibition of TCF7 by plant miR-159 could result in the reduced expression of the c-MYC oncoprotein (Fig. 2B). If confirmed, this phenomenon would expand the relevance of dietary plant-derived miRNAs to tumor prevention. Furthermore, a non-gastrointestinal tumor appears to be affected by dietary plant-derived xeno-miRNAs that would argue for the systemic relevance of these epigenetic modulators.
Figure 2.
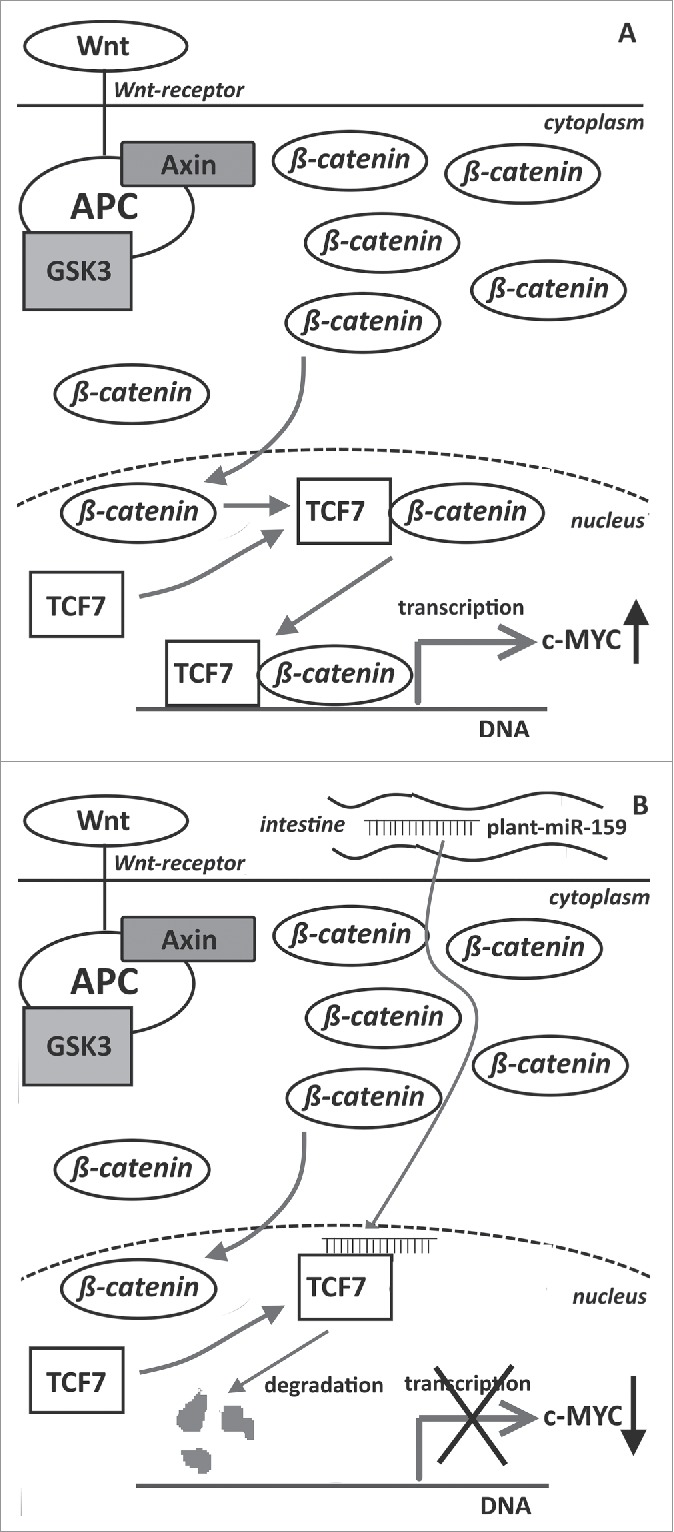
Schematic representation of the antitumor activity of plant miR-159 based on the results of Chin et al.77 (A) relevance of Wnt-signaling, TCF7 and c-myc in breast tumor progression, (B) plant-derived miR-159 might inhibit tumor progression by inhibiting TCF7
In another hypothesis, we supposed that the tissue specific action of miRNA might actually present a defense mechanism against the tumor-modulating role of circulating miRNA. As tumors are known to secrete various miRNA that are able to modulate various nearby and even distant cells, the tissue specific action of miRNA would prevent that a certain miRNA set would exert the same gene expression modulating activity in various tissues.110
Among further diseases where miRNA might be relevant, we could highlight cardiovascular diseases. As presented in the first, ground-breaking study on dietary miRNA, rice miR-168a affected the expression of a cholesterol-modulating enzyme. Although this finding is intensively debated, the modulation of lipid homeostasis by miRNA remains a major research question. Is it possible that ingested miRNA affect lipid homeostasis and thus cardiovascular morbidity? We could even hypothesize that dietary miRNA might be involved in the background of the French-paradox, as well.111 The French-paradox relates to the epidemiological observation that cardiovascular morbidity and mortality rates are much better in Southern France compared to other industrialized countries such as the UK or USA.112,113 Consumption of red wine and its antioxidant activity is most widely accepted as a dietary explanation for this phenomenon, but we cannot exclude that grape-derived miRNA might also have a role.
miRNAs as epigenetic mediators acting in cross-kingdom fashion, “epigenetic linkers”
Regarding the potential relevance of miRNAs in the inter-individual and inter-species epigenetic communications, we have hypothesized that as most miRNA genes are found in the non-coding part of the genome, one of the functions of this functionally obscure “dark matter of the genome” might be related to the regulation of epigenetic communication.30
If plant miRNA would really have relevant biological activity in animals, miRNAs could be regarded as general mediators acting as “epigenetic linkers” between species. It cannot even be excluded that animal miRNA might affect plants, as well, i.e. miRNA excreted in stool or urine might affect plants if these could be absorbed from soil. There are, however, no experimental findings whatsoever to support such interactions at present, but the potential of miRNAs acting in a cross-kingdom fashion is rather intriguing, and if valid, this hypothesis could be somewhat analogous to the former “Gaia-hypothesis."114 The intensively debated Gaia-hypothesis claimed that organisms interact with each other and the inorganic world to form a complex self-regulating system that is important for earth habitability.115 Whereas the Gaia-hypothesis also assumed interactions between the living and non-living world, miRNAs would only act among living creatures. miRNAs could wander via the food chain from plants to animals, and we hypothesized that “master regulatory” miRNAs having targets in various species might also exist.30 (Fig. 3)
Figure 3.
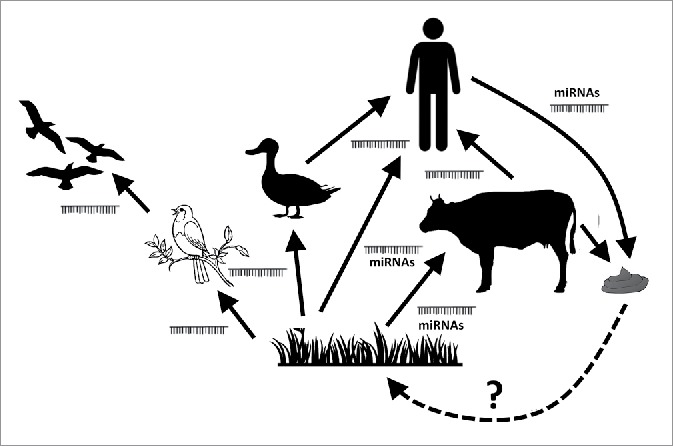
miRNAs as epigenetic linkers between different species wandering via the food chain. There are experimental data on the transfer pf xeno-miRNAs between plants and animals, and among animals, but there are no data yet of animal miRNAs affecting plants. This purely hypothetic connection between animals and plants potentially via stool miRNAs released to the soil is represented by a dashed arrow.
Treatment potential for xeno-miRNAs
The dysregulation of miRNAs is implicated in several pathological conditions116 and restoration of the normal miRNA homeostasis can be regarded as a major treatment goal. In cancer therapy, reducing the activity of oncogenic miRNAs by miRNA antagonists (anti-miRs) or enhancing tumor suppressor miRNAs is intensively investigated.117 There are promising results for both approaches.118,119,120 Apart from the treatment-oriented intraspecies modulation of miRNAs, the potential for xeno-miRNA in treatment might also be raised.
In addition to the aforementioned study reporting on the potential anti-tumor effect of plant-derived miR-159 in breast cancer,106 there are other findings suggesting the potential utility of xeno-miRNAs in medical therapy.121,122 A recent study revealed that the long-known beneficial effect of honeysuckle decoction (Lonicera japonica) traditionally used in Chinese medicine against flu might imply miRNA. A rRNA-derived-atypical miRNA, miR-2911 has been identified that is resistant to boiling and can reach an adequate amount in the honeysuckle decoction. miR-2911 targets some types of influenza A viruses and is able to obstruct the replication of the virus123 causing a significantly lower mortality in an influenza infected mice model. miR-2911 and its synthetic variant have been shown to accumulate in the lung of mice via microvesicles, suggesting that miR-2911 can be absorbed and encapsulated in microvesicles by intestinal epithelial cells.124,125
In another very recent study,122 3 known tumor suppressor miRNAs (miR-34a, miR-143 and miR-145) acting as tumor suppressor in colon cancer, and biotechnologically engineered to be methylated in a plant miRNA fashion have been investigated.81,126,127 The plant mimic miRNAs resulted in a massive reduction of tumor progression, moreover, this study has also provided evidence for the active uptake of plant-structured miRNAs in mammals.78,122
These findings could be even more intriguing by considering the fact that more than half of the world's population consumes plants as primarily nutrients and one-sixth of them suffer from gastrointestinal or chronic kidney diseases that might further affect the absorption and distribution of miRNA.128
Biotechnologically engineered plants producing miRNAs able to recognize human gene sequences might thus represent a novel direction in therapy, but a very intensive experimental and biotechnological work-up will be needed for this to be realized.
Conclusions
The potential of inter-species gene expression modulating activity of miRNA represents one of the most exciting and intriguing fields of contemporary biology and medicine. The notion that xeno-miRNA from evolutionarily distant species, such as plants, viruses, other animals might affect gene expression in the host might expand the range of factors able to interfere with endogenous gene expression, and thus disease pathogenesis. Although there are no clear, irrefutable findings at present, and several controversial findings have been produced, the potential for these interactions warrants further investigations, and also might open novel perspectives in other developing fields, such as xenotransplantation.129 Beside their potential pathogenic relevance, xeno-miRNA might even open new perspectives in medical therapy, as well.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study has been supported by a grant from the Hungarian National Research, Development and Innovation Office (NKFIH K115398) to Dr. Peter Igaz.
References
- 1.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004; 5:522-31; PMID:15211354; http://dx.doi.org/ 10.1038/nrg1379 [DOI] [PubMed] [Google Scholar]
- 2.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev 2009; 23:781-3; PMID:19339683; http://dx.doi.org/ 10.1101/gad.1787609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malumbres M. miRNAs and cancer: An epigenetics view. Mol Aspects Med 2013; 34:863-74; PMID:22771542; http://dx.doi.org/ 10.1016/j.mam.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabris L, Calin GA. Circulating free xeno-microRNAs - The new kids on the block. Mol Oncol 2016; 10:503-8; PMID:26860056; http://dx.doi.org/ 10.1016/j.molonc.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 2004; 23:4051-60; PMID:15372072; http://dx.doi.org/ 10.1038/sj.emboj.7600385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004; 432:235-40; PMID:15531877; http://dx.doi.org/ 10.1038/nature03120 [DOI] [PubMed] [Google Scholar]
- 7.Takeiwa T, Taniguchi I, Ohno M. Exportin-5 mediates nuclear export of SRP RNA in vertebrates. Genes Cells 2015; 20:281-91; PMID:25656399; http://dx.doi.org/ 10.1111/gtc.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meijer HA, Smith EM, Bushell M. Regulation of miRNA strand selection: follow the leader? Biochem Soc Trans 2014; 42:1135-40; PMID:25110015; http://dx.doi.org/ 10.1042/BST20140142 [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215-33; PMID:19167326; http://dx.doi.org/ 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 2008; 9:102-14; PMID:18197166; http://dx.doi.org/ 10.1038/nrg2290 [DOI] [PubMed] [Google Scholar]
- 11.Klopfleisch R, Weiss ATA, Gruber AD. Excavation of a buried treasure–DNA, mRNA, miRNA and protein analysis in formalin fixed, paraffin embedded tissues. Histol Histopathol 2011; 26:797-810; PMID:21472693; http://dx.doi.org/ 10.14670/HH-26.797 [DOI] [PubMed] [Google Scholar]
- 12.Axtell MJ, Westholm JO, Lai EC. Vive la différence: biogenesis and evolution of microRNAs in plants and animals. Genome Biol 2011; 12:221; PMID:21554756; http://dx.doi.org/ 10.1186/gb-2011-12-4-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007; 318:1931-4; PMID:18048652; http://dx.doi.org/ 10.1126/science.1149460 [DOI] [PubMed] [Google Scholar]
- 14.Fabbri M, Ivan M, Cimmino A, Negrini M, Calin GA. Regulatory mechanisms of microRNAs involvement in cancer. Expert Opin Biol Ther 2007; 7:1009-19; PMID:17665990; http://dx.doi.org/ 10.1517/14712598.7.7.1009 [DOI] [PubMed] [Google Scholar]
- 15.Perge P, Nagy Z, Igaz I, Igaz P. Suggested roles for microRNA in tumors. Biomol Concepts 2015; 6:149-55; PMID:25870972; http://dx.doi.org/ 10.1515/bmc-2015-0002 [DOI] [PubMed] [Google Scholar]
- 16.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med 2005; 353:1768-71; PMID:16251533; http://dx.doi.org/ 10.1056/NEJMp058190 [DOI] [PubMed] [Google Scholar]
- 17.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem 2010; 56:1733-41; PMID:20847327; http://dx.doi.org/ 10.1373/clinchem.2010.147405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redis RS, Calin S, Yang Y, You MJ, Calin GA. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacol Ther 2012; 136:169-74; PMID:22903157; http://dx.doi.org/ 10.1016/j.pharmthera.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid G, Kirschner MB, van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit Rev Oncol Hematol 2011; 80:193-208; PMID:21145252; http://dx.doi.org/ 10.1016/j.critrevonc.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 20.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 2010; 5:e11803; PMID:20668554; http://dx.doi.org/ 10.1371/journal.pone.0011803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, Hastings ML, Duelli DM. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One 2010; 5:e13515; PMID:20976003; http://dx.doi.org/ 10.1371/journal.pone.0013515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guduric-Fuchs J, O'Connor A, Camp B, O'Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics 2012; 13:357; PMID:22849433; http://dx.doi.org/ 10.1186/1471-2164-13-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turchinovich A, Tonevitsky AG, Burwinkel B. Extracellular miRNA: A Collision of Two Paradigms. Trends Biochem Sci 2016; 41:883-92; PMID:27597517; http://dx.doi.org/ 10.1016/j.tibs.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 24.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, et al.. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 2008; 3:e3694; PMID:19002258; http://dx.doi.org/ 10.1371/journal.pone.0003694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 2011; 13:423-33; PMID:21423178; http://dx.doi.org/ 10.1038/ncb2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res 2011; 1:98-110; PMID:21969178; [PMC free article] [PubMed] [Google Scholar]
- 27.Katsuda T, Kosaka N, Ochiya T. The roles of extracellular vesicles in cancer biology: toward the development of novel cancer biomarkers. Proteomics 2014; 14:412-25; PMID:24339442; http://dx.doi.org/ 10.1002/pmic.201300389 [DOI] [PubMed] [Google Scholar]
- 28.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, et al.. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25:501-15; PMID:24735924; http://dx.doi.org/ 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat Rev Clin Oncol 2011; 8:467-77; PMID:21647195; http://dx.doi.org/ 10.1038/nrclinonc.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Igaz I, Igaz P. Possible role for microRNAs as inter-species mediators of epigenetic information in disease pathogenesis: is the non-coding dark matter of the genome responsible for epigenetic interindividual or interspecies communication? Med Hypotheses 2015; 84:150-4; PMID:25535106; http://dx.doi.org/ 10.1016/j.mehy.2014.11.021 [DOI] [PubMed] [Google Scholar]
- 31.Turchinovich A, Burwinkel B. Distinct AGO1 and AGO2 associated miRNA profiles in human cells and blood plasma. RNA Biol 2012; 9:1066-75; PMID:22858679; http://dx.doi.org/ 10.4161/rna.21083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, et al.. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A 2014; 111:14888-93; PMID:25267620; http://dx.doi.org/ 10.1073/pnas.1408301111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ, et al.. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011; 7:780-8; PMID:21601655; http://dx.doi.org/ 10.1016/j.nano.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiland M, Weiland M, Gao XH, Zhou L, Mi QS. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol 2012; 9:850-9; PMID:22699556; http://dx.doi.org/ 10.4161/rna.20378 [DOI] [PubMed] [Google Scholar]
- 35.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol 2013; 191:6250-60; PMID:24227773; http://dx.doi.org/ 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patton JG, Franklin JL, Weaver AM, Vickers K, Zhang B, Coffey RJ, Ansel KM, Blelloch R, Goga A, Huang B, et al.. Biogenesis, delivery, and function of extracellular RNA. J Extracell vesicles 2015; 4:27494; PMID:26320939; http://dx.doi.org/ 10.3402/jev.v4.27494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, et al.. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A 2012; 109:E2110-6; PMID:22753494; http://dx.doi.org/ 10.1073/pnas.1209414109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alsaweed M, Hartmann PE, Geddes DT, Kakulas F. MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. Int J Environ Res Public Health 2015; 12:13981-4020; PMID:26529003; http://dx.doi.org/ 10.3390/ijerph121113981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al.. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18:997-1006; PMID:18766170; http://dx.doi.org/ 10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 40.Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M, Admyre C, Johansson SM, Qazi KR, Filén JJ, et al.. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci 2012; 95:4831-41; PMID:22916887; http://dx.doi.org/ 10.3168/jds.2012-5489 [DOI] [PubMed] [Google Scholar]
- 41.Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci Rep 2016; 6:20680; PMID:26854194; http://dx.doi.org/ 10.1038/srep20680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melnik BC, Kakulas F, Geddes DT, Hartmann PE, John SM, Carrera-Bastos P, Cordain L, Schmitz G. Milk miRNAs: simple nutrients or systemic functional regulators? Nutr Metab 2016; 13:42; PMID:27330539; http://dx.doi.org/ 10.1186/s12986-016-0101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010; 1:7; PMID:20226005; http://dx.doi.org/ 10.1186/1758-907X-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Guillou S, Sdassi N, Laubier J, Passet B, Vilotte M, Castille J, Laloë D, Polyte J, Bouet S, Jaffrézic F, et al.. Overexpression of miR-30b in the developing mouse mammary gland causes a lactation defect and delays involution. PLoS One 2012; 7:e45727; PMID:23029204; http://dx.doi.org/ 10.1371/journal.pone.0045727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Title AC, Denzler R, Stoffel M. Uptake and Function Studies of Maternal Milk-derived MicroRNAs. J Biol Chem 2015; 290:23680-91; PMID:26240150; http://dx.doi.org/ 10.1074/jbc.M115.676734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naqvi AR, Fordham JB, Nares S. miR-24, miR-30b, and miR-142-3p regulate phagocytosis in myeloid inflammatory cells. J Immunol 2015; 194:1916-27;PMID:25601927; http://dx.doi.org/ 10.4049/jimmunol.1401893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laubier J, Castille J, Le Guillou S, Le Provost F. No effect of an elevated miR-30b level in mouse milk on its level in pup tissues. RNA Biol 2015; 12:26-9; PMID:25763824; http://dx.doi.org/ 10.1080/15476286.2015.1017212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopp F, Wagner E, Roidl A. The proto-oncogene KRAS is targeted by miR-200c. Oncotarget 2014; 5:185-95; PMID:24368337; http://dx.doi.org/ 10.18632/oncotarget.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salunkhe VA, Esguerra JL, Ofori JK, Mollet IG, Braun M, Stoffel M, Wendt A, Eliasson L. Modulation of microRNA-375 expression alters voltage-gated Na(+) channel properties and exocytosis in insulin-secreting cells. Acta Physiol (Oxf) 2015; 213:882-92; PMID:25627423; http://dx.doi.org/ 10.1111/apha.12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherji S, Ebert MS, Zheng GX, Tsang JS, Sharp PA, van Oudenaarden A. MicroRNAs can generate thresholds in target gene expression. Nat Genet 2011; 43:854-9; PMID:21857679; http://dx.doi.org/ 10.1038/ng.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mullokandov G, Baccarini A, Ruzo A, Jayaprakash AD, Tung N, Israelow B, Evans MJ, Sachidanandam R, Brown BD. High-throughput assessment of microRNA activity and function using microRNA sensor and decoy libraries. Nat Methods 2012; 9:840-6; PMID:22751203; http://dx.doi.org/ 10.1038/nmeth.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess 2007; 1-186; PMID:17764214; [PMC free article] [PubMed] [Google Scholar]
- 53.Melnik BC, John SM, Carrera-Bastos P, Schmitz G. Milk: a postnatal imprinting system stabilizing FoxP3 expression and regulatory T cell differentiation. Clin Transl Allergy 2016; 6:18; PMID:27175277; http://dx.doi.org/ 10.1186/s13601-016-0108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melnik BC, John SM, Schmitz G. Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J Transl Med 2014; 12:43; PMID:24521175; http://dx.doi.org/ 10.1186/1479-5876-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep 2011; 11:139-45; PMID:21271314; http://dx.doi.org/ 10.1007/s11882-011-0178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Igaz I, Igaz P. Hypothetic Interindividual and Interspecies Relevance of microRNAs Released in Body Fluids. EXS 2015; 106:281-8; PMID:26608210; http://dx.doi.org/ 10.1007/978-3-0348-0955-9_14 [DOI] [PubMed] [Google Scholar]
- 57.Snow JW, Hale AE, Isaacs SK, Baggish AL, Chan SY. Ineffective delivery of diet-derived microRNAs to recipient animal organisms. RNA Biol 2013; 10:1107-16; PMID:23669076; http://dx.doi.org/ 10.4161/rna.24909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf T, Baier SR, Zempleni J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J Nutr 2015; 145:2201-6; PMID:26269243; http://dx.doi.org/ 10.3945/jn.115.218586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Izumi H, Tsuda M, Sato Y, Kosaka N, Ochiya T, Iwamoto H, Namba K, Takeda Y, Argyropoulos C, Wang K, et al.. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J Dairy Sci 2015; 98:2920-33; PMID:25726110; http://dx.doi.org/ 10.3168/jds.2012-5489 [DOI] [PubMed] [Google Scholar]
- 60.Kusuma RJ, Manca S, Friemel T, Sukreet S, Nguyen C, Zempleni J, Abu-Halima M, Hammadeh M, Schmitt J, Leidinger P, et al.. Human vascular endothelial cells transport foreign exosomes from cow's milk by endocytosis. Am J Physiol Cell Physiol 2016; 310:C800-7; PMID:26984735; http://dx.doi.org/ 10.1152/ajpcell.00169.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howard KM, Jati Kusuma R, Baier SR, Friemel T, Markham L, Vanamala J, Zempleni J. Loss of miRNAs during processing and storage of cow's (Bos taurus) milk. J Agric Food Chem 2015; 63:588-92; PMID:25565082; http://dx.doi.org/ 10.1021/jf505526w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucey JA. Raw Milk Consumption: Risks and Benefits. Nutr Today 2015; 50:189-93; PMID:27340300; http://dx.doi.org/ 10.1097/NT.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr 2014; 144:1495-500; PMID:25122645; http://dx.doi.org/ 10.3945/jn.114.196436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Witwer KW. Diet-responsive mammalian miRNAs are likely endogenous. J Nutr 2014; 144:1880-1; PMID:25332488; http://dx.doi.org/ 10.3945/jn.114.202523 [DOI] [PubMed] [Google Scholar]
- 65.Baier SR, Xie F, Zempleni J. Reply to Witwer. J Nutr 2014; 144:1882; PMID:25332489; http://dx.doi.org/ 10.3945/jn.114.202606 [DOI] [PubMed] [Google Scholar]
- 66.Griffiths-Jones S. miRBase: microRNA Sequences and Annotation. In: Current Protocols in Bioinformatics. 2010; 12.9.1:12.9.10; PMID:20205188, http://dx.doi.org/ 10.1002/0471250953.bi1209s29 [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 2009; 284:15676-84; PMID:19342382; http://dx.doi.org/ 10.1074/jbc.M809787200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossi M, Pitari MR, Amodio N, Di Martino MT, Conforti F, Leone E, Botta C, Paolino FM, Del Giudice T, Iuliano E, et al.. miR-29b negatively regulates human osteoclastic cell differentiation and function: Implications for the treatment of multiple myeloma-related bone disease. J Cell Physiol 2013; 228:1506-15; PMID:23254643; http://dx.doi.org/ 10.1002/jcp.24306 [DOI] [PubMed] [Google Scholar]
- 69.Melnik BC. The pathogenic role of persistent milk signaling in mTORC1- and milk-microRNA-driven type 2 diabetes mellitus. Curr Diabetes Rev 2015; 11:46-62; PMID:25587719; http://dx.doi.org/ 10.2174/1573399811666150114100653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melnik BC, John SM, Schmitz G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr J 2013; 12:103; PMID:23883112; http://dx.doi.org/ 10.1186/1475-2891-12-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lei BX, Liu ZH, Li ZJ, Li C, Deng Y-F. miR-21 induces cell proliferation and suppresses the chemosensitivity in glioblastoma cells via downregulation of FOXO1. Int J Clin Exp Med 2014; 7:2060-6; PMID:25232387 [PMC free article] [PubMed] [Google Scholar]
- 72.Mersey BD, Jin P, Danner DJ. Human microRNA (miR29b) expression controls the amount of branched chain alpha-ketoacid dehydrogenase complex in a cell. Hum Mol Genet 2005; 14:3371-7; PMID:16203741; http://dx.doi.org/ 10.1093/hmg/ddi368 [DOI] [PubMed] [Google Scholar]
- 73.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 2008; 283:14910-4; PMID:18411277; http://dx.doi.org/ 10.1074/jbc.C800074200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 2008; 9:582-9; PMID:18483486; http://dx.doi.org/ 10.1038/embor.2008.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Auerbach A, Vyas G, Li A, Halushka M, Witwer K. Uptake of dietary milk miRNAs by adult humans: a validation study. F1000Research 2016; 5:721; PMID:27158459; http://dx.doi.org/ 10.12688/f1000research.8548.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of Dairy Products, Calcium, and Vitamin D and Risk of Breast Cancer. CancerSpectrum Knowl Environ 2002; 94:1301-10; PMID:12208895; http://dx.doi.org/ 10.1093/jnci/94.17.1301 [DOI] [PubMed] [Google Scholar]
- 77.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, et al.. Dairy Foods, Calcium, and Colorectal Cancer: A Pooled Analysis of 10 Cohort Studies. JNCI J Natl Cancer Inst 2004; 96:1015-22; PMID:15240785; http://dx.doi.org/ 10.1093/jnci/djh185 [DOI] [PubMed] [Google Scholar]
- 78.Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, Li J, Bian Z, Liang X, Cai X, et al.. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 2012; 22:107-26; PMID:21931358; http://dx.doi.org/ 10.1038/cr.2011.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Witwer KW. XenomiRs and miRNA homeostasis in health and disease: evidence that diet and dietary miRNAs directly and indirectly influence circulating miRNA profiles. RNA Biol 2012; 9:1147-54; PMID:22951590; http://dx.doi.org/ 10.4161/rna.21619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Semenov DV, Baryakin DN, Brenner EV, Kurilshikov AM, Vasiliev GV, Bryzgalov LA, Chikova ED, Filippova JA, Kuligina EV, Richter VA. Unbiased approach to profile the variety of small non-coding RNA of human blood plasma with massively parallel sequencing technology. Expert Opin Biol Ther 2012; 12 Suppl 1:S43-51; PMID:22509727; http://dx.doi.org/ 10.1517/14712598.2012.679653 [DOI] [PubMed] [Google Scholar]
- 81.Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, Steward R, Chen X. Methylation as a crucial step in plant microRNA biogenesis. Science 2005; 307:932-5; PMID:15705854; http://dx.doi.org/ 10.1126/science.1107130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang H, Huang L, Cao J, Zen K, Chen X, Zhang CY. Regulation of mammalian gene expression by exogenous microRNAs. Wiley Interdiscip Rev RNA 2012; 3:733-42; PMID:22740375, http://dx.doi.org/ 10.1002/wrna.1127 [DOI] [PubMed] [Google Scholar]
- 83.Wilund KR, Yi M, Campagna F, Arca M, Zuliani G, Fellin R, Ho YK, Garcia JV, Hobbs HH, Cohen JC. Molecular mechanisms of autosomal recessive hypercholesterolemia. Hum Mol Genet 2002; 11:3019-30; PMID:12417523, http://dx.doi.org/ 10.1093/hmg/11.24.3019 [DOI] [PubMed] [Google Scholar]
- 84.Verhoeven V, Van der Auwera A, Van Gaal L, Remmen R, Apers S, Stalpaert M, Wens J, Hermans N. Can red yeast rice and olive extract improve lipid profile and cardiovascular risk in metabolic syndrome?: A double blind, placebo controlled randomized trial. BMC Complement Altern Med 2015; 15:52; PMID:25879228; http://dx.doi.org/ 10.1186/s12906-015-0576-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jolfaie NR, Rouhani MH, Surkan PJ, Siassi F, Azadbakht L. Rice Bran Oil Decreases Total and LDL Cholesterol in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Horm Metab Res 2016; 48:417-26; PMID:27311126; http://dx.doi.org/ 10.1055/s-0042-105748 [DOI] [PubMed] [Google Scholar]
- 86.Hirschi KD. New foods for thought. Trends Plant Sci 2012; 17:123-5;. PMID:22265093; http://dx.doi.org/ 10.1016/j.tplants.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 87.Witwer KW, Hirschi KD. Hirschi KD. Transfer and functional consequences of dietary microRNAs in vertebrates: concepts in search of corroboration: negative results challenge the hypothesis that dietary xenomiRs cross the gut and regulate genes in ingesting vertebrates, but important questions persist. Bioessays 2014; 36:394-406; PMID:24436255; http://dx.doi.org/ 10.1002/bies.201300150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Wiggins BE, Lawrence C, Petrick J, Ivashuta S, Heck G. Analysis of plant-derived miRNAs in animal small RNA datasets. BMC Genomics 2012; 13:381; PMID:22873950; http://dx.doi.org/ 10.1186/1471-2164-13-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lukasik A, Zielenkiewicz P. In silico identification of plant miRNAs in mammalian breast milk exosomes–a small step forward? PLoS One 2014; 9:e99963; PMID:24933019; http://dx.doi.org/ 10.1371/journal.pone.0099963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bağcı C, Allmer J. One Step Forward, Two Steps Back; Xeno-MicroRNAs Reported in Breast Milk Are Artifacts. PLoS One 2016; 11:e0145065; PMID:26824347; http://dx.doi.org/ 10.1371/journal.pone.0145065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Witwer KW, McAlexander MA, Queen SE, Adams RJ. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs: limited evidence for general uptake of dietary plant xenomiRs. RNA Biol 2013; 10:1080-6; PMID:23770773; http://dx.doi.org/ 10.4161/rna.25246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams Z, Ben-Dov IZ, Elias R, Mihailovic A, Brown M, Rosenwaks Z, Tuschl T. Comprehensive profiling of circulating microRNA via small RNA sequencing of cDNA libraries reveals biomarker potential and limitations. Proc Natl Acad Sci U S A 2013; 110:4255-60; PMID:23440203; http://dx.doi.org/ 10.1073/pnas.1214046110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, et al.. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007; 129:1401-14; PMID:17604727; http://dx.doi.org/ 10.1016/j.cell.2007.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brown BD, Gentner B, Cantore A, Colleoni S, Amendola M, Zingale A, Baccarini A, Lazzari G, Galli C, Nadini L. Endogenous microRNA can be broadly exploited to regulatetransgene expression according to tissue, lineage and differentiation state. Nat Biotechnol 2007;25:1457-67; PMID:18026085; http://dx.doi.org/ 10.1038/nbt1372 [DOI] [PubMed] [Google Scholar]
- 95.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:806-11;PMID:9486653; http://dx.doi.org/ 10.1038/35888 [DOI] [PubMed] [Google Scholar]
- 96.Mello CC, Conte D. Revealing the world of RNA interference. Nature 2004; 431:338-42; PMID:15372040; http://dx.doi.org/ 10.1038/nature02872 [DOI] [PubMed] [Google Scholar]
- 97.Whangbo JS, Hunter CP. Environmental RNA interference. Trends Genet 2008; 24:297-305; PMID:18450316; http://dx.doi.org/ 10.1016/j.tig.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 98.Winston WM, Molodowitch C, Hunter CP. Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 2002; 295:2456-9; PMID:11834782; http://dx.doi.org/ 10.1126/science.1068836 [DOI] [PubMed] [Google Scholar]
- 99.Winston WM, Sutherlin M, Wright AJ, Feinberg EH, Hunter CP. Caenorhabditis elegans SID-2 is required for environmental RNA interference. Proc Natl Acad Sci U S A 2007; 104:10565-70; PMID:17563372; http://dx.doi.org/ 10.1073/pnas.0611282104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McEwan DL, Weisman AS, Hunter CP. Uptake of extracellular double-stranded RNA by SID-2. Mol Cell 2012; 47:746-54;. PMID:22902558; http://dx.doi.org/ 10.1016/j.molcel.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duxbury MS, Ashley SW, Whang EE. RNA interference: a mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem Biophys Res Commun 2005; 331:459-63;. PMID:15850781; http://dx.doi.org/ 10.1016/j.bbrc.2005.03.199 [DOI] [PubMed] [Google Scholar]
- 102.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, et al.. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol 2007; 25:1149-57; PMID:17873866; http://dx.doi.org/ 10.1038/nbt1339 [DOI] [PubMed] [Google Scholar]
- 103.Elhassan MO, Christie J, Duxbury MS. Homo sapiens systemic RNA interference-defective-1 transmembrane family member 1 (SIDT1) protein mediates contact-dependent small RNA transfer and microRNA-21-driven chemoresistance. J Biol Chem 2012; 287:5267-77; PMID:22174421; http://dx.doi.org/ 10.1074/jbc.m111.318865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fabbri M. TLRs as miRNA receptors. Cancer Res 2012; 72:6333-7; PMID:23222301; http://dx.doi.org/ 10.1158/0008-5472.can-12-3229 [DOI] [PubMed] [Google Scholar]
- 105.Igaz I, Igaz P. Tumor surveillance by circulating microRNAs: a hypothesis. Cell Mol Life Sci 2014; 71:4081-7; PMID:25037157; http://dx.doi.org/ 10.1007/s00018-014-1682-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chin AR, Fong MY, Somlo G, Wu J, Swiderski P, Wu X, Wang SE. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res 2016; 26:217-28; PMID:26794868; http://dx.doi.org/ 10.1038/cr.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121:2750-67; PMID:21633166; http://dx.doi.org/ 10.1172/jci45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bilir B, Kucuk O, Moreno CS. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J Transl Med 2013; 11:280; PMID:24188694; http://dx.doi.org/ 10.1186/1479-5876-11-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnson JP, Kumar P, Koulnis M, Patel M, Simin K. Crucial and novel cancer drivers in a mouse model of triple-negative breast cancer. Cancer Genomics Proteomics 2014; 11:115-26; PMID:24969692 [PubMed] [Google Scholar]
- 110.Igaz I, Igaz P. Why is microRNA action tissue specific? A putative defense mechanism against growth disorders, tumor development or progression mediated by circulating microRNA? Med Hypotheses 2015; 85:530-3; PMID:26198739; http://dx.doi.org/ 10.1016/j.mehy.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 111.Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol 2012; 3:141; PMID:22822401; http://dx.doi.org/ 10.3389/fphar.2012.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992; 339:1523-6; PMID:1351198; http://dx.doi.org/ 10.1016/0140-6736(92)91277-f [DOI] [PubMed] [Google Scholar]
- 113.Criqui M., Ringel B. Does diet or alcohol explain the French paradox? Lancet 1994; 344:1719-23; PMID:7996999; http://dx.doi.org/ 10.1016/s0140-6736(94)92883-5 [DOI] [PubMed] [Google Scholar]
- 114.Karnani M, Annila A. Gaia again. Biosystems 2009; 95:82-7; PMID:18706969; http://dx.doi.org/ 10.1016/j.biosystems.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 115.Lovelock JE. New statements on the Gaia theory. Microbiol 1995; 11:295-304; PMID:7576345 [PubMed] [Google Scholar]
- 116.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer 2010; 10:389-402; PMID:20495573; http://dx.doi.org/ 10.1038/nrc2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McDermott AM, Heneghan HM, Miller N, Kerin MJ. The therapeutic potential of microRNAs: disease modulators and drug targets. Pharm Res 2011; 28:3016-29;PMID:21818713; http://dx.doi.org/ 10.1007/s11095-011-0550-2 [DOI] [PubMed] [Google Scholar]
- 118.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, et al.. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 2009; 137:1005-17;. PMID:19524505; http://dx.doi.org/ 10.1016/j.cell.2009.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res 2010; 70:5923-30; PMID:20570894; http://dx.doi.org/ 10.1158/0008-5472.can-10-0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, et al.. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013; 368:1685-94; PMID:23534542; http://dx.doi.org/ 10.1056/nejmoa1209026 [DOI] [PubMed] [Google Scholar]
- 121.Zhou Z, Li X, Liu J, Dong L, Chen Q, Liu J, Kong H, Zhang Q, Qi X, Hou D, et al.. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res 2015; 25:39-49; PMID:25287280; http://dx.doi.org/ 10.1038/cr.2014.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mlotshwa S, Pruss GJ, MacArthur JL, Endres MW, Davis C, Hofseth LJ, Peña MM, Vance V. A novel chemopreventive strategy based on therapeutic microRNAs produced in plants. Cell Res 2015; 25:521-4; PMID:25721325; http://dx.doi.org/ 10.1038/cr.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang MS, Law FC, Wong RN, Mak NK, Wei XY. Interaction between oseltamivir and herbal medicines used for treating avian influenza. Hong Kong Med J 2012; 18 Suppl 6:34-6;. PMID:23249852; [PubMed] [Google Scholar]
- 124.Yang J, Farmer LM, Agyekum AA, Hirschi KD. Detection of dietary plant-based small RNAs in animals. Cell Res 2015; 25:517-20; PMID:25721324; http://dx.doi.org/ 10.1038/cr.2015.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang J, Farmer LM, Agyekum AA, Elbaz-Younes I, Hirschi KD. Detection of an abundant plant-based small RNA in healthy consumers. PLoS One 2015; 10:e0137516. PMID:26335106; http://dx.doi.org/ 10.1371/journal.pone.0137516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet 2012; 3:120; PMID:22783274; http://dx.doi.org/ 10.3389/fgene.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takaoka Y, Shimizu Y, Hasegawa H, Ouchi Y, Qiao S, Nagahara M, Ichihara M, Lee JD, Adachi K, Hamaguchi M, et al.. Forced expression of miR-143 represses ERK5/c-Myc and p68/p72 signaling in concert with miR-145 in gut tumors of Apc(Min) mice. PLoS One 2012; 7:e42137. PMID:22876303; http://dx.doi.org/ 10.1371/journal.pone.0042137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bischoff SC. “Gut health”: a new objective in medicine? BMC Med 2011; 9:24;. PMID:21401922; http://dx.doi.org/ 10.1186/1741-7015-9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Igaz P. Recent strategies to overcome the hyperacute rejection in pig to human xenotransplantation. Yale J Biol Med 2001; 74:329-40; PMID:11769339 [PMC free article] [PubMed] [Google Scholar]


