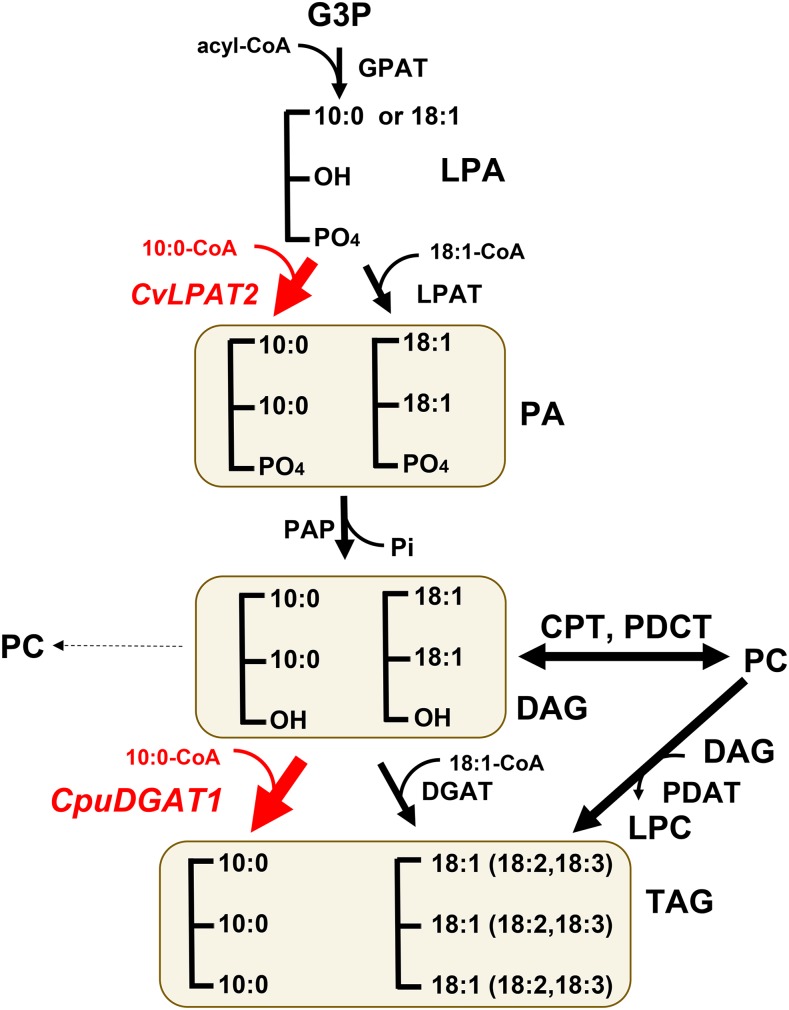
Figure 10.
A proposed pathway for 10:0-rich TAG assembly in transgenic camelina seeds coexpressing CvLPAT2 and CpuDGAT1. Acyl-CoA species of 10:0 and 18:1 are sequentially esterified to glycerol-3-P (G3P) at sn-1 and sn-2 positions by GPAT and LPAT, forming LPA and PA. The release of P from PA by phosphatidic acid phosphatase forms DAG. The subsequent acylation of DAG at sn-3 catalyzed by DGAT forms TAG. DAG can be converted to PC via the action of DAG/CPT and/or PDCT. DAG molecules containing 10:0 at the sn-2 position arise from the specialized activity of the Cuphea LPAT CvLPAT2. DAGs containing 10:0 at the sn-2 position are selectively used for acylation with 10:0-CoA at the sn-3 position by activity of the specialized Cuphea diacylglycerol acyltransferase CpuDGAT1 to form TAG species enriched in 10:0. DAG molecules containing 10:0 appear to be poor substrates for PC synthesis, or 10:0 is rapidly removed from PC formed from 10:0-DAG species. As such, 10:0 accumulation in TAG appears to proceed primarily by the Kennedy pathway rather than through PC via activity of enzymes including PDCT and phospholipid/diacylglycerol acyltransferase, which catalyzes the transfer of fatty acids at the sn-2 position of PC to the sn-3 position of DAG producing TAG and lysophosphatidylcholine. The PC route of fatty acid flux for TAG synthesis involves primarily flux of 18:1 for desaturation to 18:2 and 18:3. LPC, lysophosphatidylcholine; PAP, phosphatidic acid phosphatase; PDAT, phospholipid/diacylglycerol acyltransferase.