We propose a unified nomenclature of an important group of plant chromatin proteins, based on evolutionary relationships of their common nucleosome recognition element, a linker histone-type globular domain (GH1).
Abstract
H1 (or linker) histones are basic nuclear proteins that possess an evolutionarily conserved nucleosome-binding globular domain, GH1. They perform critical functions in determining the accessibility of chromatin DNA to trans-acting factors. In most metazoan species studied so far, linker histones are highly heterogenous, with numerous nonallelic variants cooccurring in the same cells. The phylogenetic relationships among these variants as well as their structural and functional properties have been relatively well established. This contrasts markedly with the rather limited knowledge concerning the phylogeny and structural and functional roles of an unusually diverse group of GH1-containing proteins in plants. The dearth of information and the lack of a coherent phylogeny-based nomenclature of these proteins can lead to misunderstandings regarding their identity and possible relationships, thereby hampering plant chromatin research. Based on published data and our in silico and high-throughput analyses, we propose a systematization and coherent nomenclature of GH1-containing proteins of Arabidopsis (Arabidopsis thaliana [L.] Heynh) that will be useful for both the identification and structural and functional characterization of homologous proteins from other plant species.
H1s, also known as linker histones, are universal and ubiquitous components of chromatin fibers, in which they occur at an average frequency of one molecule per nucleosome (Woodcock et al., 2006). They are small basic proteins with a highly conserved central globular domain (GH1) and two less conserved and mostly unstructured tail fragments: a short (∼20 amino acids) N-terminal domain and a considerably longer (∼100 amino acids) and highly positively charged C-terminal domain (CTD). GH1 consists of ∼80 amino acids and belongs to the winged helix family of DNA-binding proteins. It contains a characteristic mixed α/β-fold consisting of three α-helices (I–III) and two β-strands (S2 and S3). The compact bundle composed of the three helices forms the core of this domain. The wing structure (from which the name of this family of DNA-binding proteins is derived) lies within the region located C terminally to helix III and is an extended loop joining β-strands S2 and S3. GH1 associates with the nucleosome outside the core particle and contacts DNA via at least two different binding sites (Zhou et al., 1998, 2013; Brown et al., 2006; Syed et al., 2010).
In addition to GH1, the overall functional properties of H1 are strongly influenced by the CTD, which binds to internucleosomal linker DNA. The CTD has an intrinsically disordered structure capable of adopting different conformations depending on the geometry of the target surfaces, which may be linker DNA or interacting proteins (Hansen et al., 2006). The prime determinant of this property is the amino acid composition rather than the CTD sequence, with charge neutralization upon DNA binding by its many Lys residues playing an important role (Hendzel et al., 2004). According to current models, simultaneous and synergistic binding of both GH1 and the CTD are prerequisites for correct H1 placement and determine its role in chromatin compaction (Stasevich et al., 2010). It is generally agreed that H1, by restricting nucleosome mobility and impeding the access of trans-acting factors to their target sequences, exerts strong effects on DNA-dependent activities, such as transcription and replication, and probably also recombination and repair (Izzo et al., 2008). Recent evidence suggests an even more complex pattern of H1 functions in the cell, in which its role as a universal architectural protein affecting chromatin dynamics is complemented by a parallel function as a local and gene-specific regulator (McBryant et al., 2010). Linker histones are a more divergent group of proteins than core histones. In animals, numerous nonallelic variants, including cell type- and stage-specific isoforms, have been described (Jerzmanowski, 2004; Sancho et al., 2008). In addition, and similar to core histones, major animal H1 variants undergo extensive posttranslational modifications of different types (Wisniewski et al., 2007), the importance of most of which is unknown.
Plant H1s exhibit the universal features of the H1 family, including the occurrence of different nonallelic variants and extensive posttranslational modifications (Table I; Supplemental Table S1; Prymakowska-Bosak et al., 1996; Jerzmanowski et al., 2000; Jerzmanowski, 2004; Kotliński et al., 2016). Interest in their functional roles has grown considerably in recent years, since they are frequently found in high-throughput screens aimed at identifying regulators involved in processes related to development, physiology, and adaptation to stresses (Wierzbicki and Jerzmanowski, 2005; She et al., 2013; Zemach et al., 2013; Over and Michaels, 2014; Rutowicz et al., 2015; Supplemental Table S2). However, because of the exceptional diversity of plant GH1-containing proteins, a fact not realized by most researchers, the relevant reference information about members of this group available in databases is highly imprecise, lacks coherence and systematization, and often is misleading, particularly for those unfamiliar with the classification of chromatin proteins. For example, as illustrated in Table I and Supplemental Table S1, plant linker histones, like high-mobility group A (HMGA) and certain other proteins, are described by the general term winged helix DNA-binding transcription factor in several databases. Numerous plant GH1-containing proteins are listed as putative or lack any description. Moreover, the annotation of the same proteins is inconsistent between databases.
Table I. Accession numbers and descriptions of Arabidopsis canonical linker histones in different databases: TAIR10, UniProt, NCBInr, and ChromDB (a copy of this discontinued database in the Web archive was used).
| Gene | Splice Variant | TAIR10 |
UniProt |
NCBInr |
ChromDB | Length | |||
|---|---|---|---|---|---|---|---|---|---|
| Identifier | Description | Identifier | Description | Identifier | Description | ||||
| amino acids | |||||||||
| H1.1 | 1 | AT1G06760.1 | Winged-helix DNA-binding transcription factor family protein | P26568 (H11_ARATH) | Histone H1.1 | NP_172161.1 | Histone H1.1, histone H1-1 | HON1 | 274 |
| P26568.1 | |||||||||
| AAF63139.1 | |||||||||
| AAL16244.1 | |||||||||
| CAA44314.1 | |||||||||
| AAK91467.1 | |||||||||
| AAM19868.1 | |||||||||
| AAM64441.1 | |||||||||
| AEE28032.1 | |||||||||
| CAA44312.1 (partial) | |||||||||
| H1.2 | 1 | AT2G30620.1 | Winged-helix DNA-binding transcription factor family protein | P26569 (H12_ARATH) | Histone H1.2 | NP_180620.1 | Histone H1.2, histone H1-2, putative histone H1 protein, histone H1 | HON2 | 273 |
| P26569.1 | |||||||||
| AAK25921.1 | |||||||||
| AAK64117.1 | |||||||||
| AEC08419.1 | |||||||||
| AAM63006.1 | |||||||||
| AAM15525.1 | |||||||||
| CAA44316.1 | |||||||||
| 2 | AT2G30620.2 | Winged-helix DNA-binding transcription factor family protein | F4INW2 | Histone H1.2 | NP_001189643.1 | Histone H1.2 | 202 | ||
| AEC08420.1 | |||||||||
| 3 | – | – | C0Z3A1 | AT2G30620 protein | BAH57180.1 | AT2G30620 | 208 | ||
| H1.3 | 1 | AT2G18050.1 | HIS1-3|histone H1-3 | P94109 | His-1-3 | histone H1 | NP_179396.1 | Histone H1-3, histone H1 | HON3 | 167 |
| AAC49789.1 | |||||||||
| AAC49790.1 | |||||||||
| AAD20121.1 | |||||||||
| AAK76471.1 | |||||||||
| AAL85145.1 | |||||||||
| AAM61167.1 | |||||||||
| AEC06720.1 | |||||||||
| 2 | AT2G18050.2 | HIS1-3|histone H1-3 | Q3EBY3 | Histone H1-3 HIS1-3 | NP_849970.1 | Histone H1-3 | 138 | ||
| AEC06721.1 | |||||||||
Here, we summarize currently available information, including both published data and the findings of our in silico and high-throughput analyses, and propose a coherent system of phylogeny and structure-based nomenclature and annotation of H1s and other GH1-containing proteins of Arabidopsis (Arabidopsis thaliana). This system will be useful as a basic reference tool for the identification and characterization of homologous proteins from different plant species. In addition, we highlight some interesting trends in the evolution of chromatin-based regulation that may be specific for plants.
RESULTS AND DISCUSSION
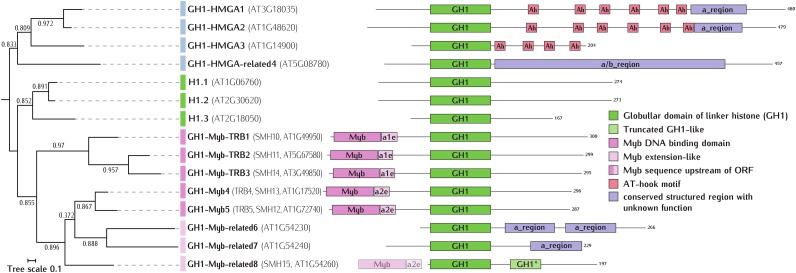
The Arabidopsis genome encodes 15 proteins containing a genuine GH1 domain. A scheme linking GH1-based phylogenetic relationships with protein domain architectures within this group is shown in Figure 1. Phylogenetic analysis supports an early separation into three subgroups, which we rename here as follows: (1) H1s; (2) GH1-HMGA/GH1-HMGA-related; and (3) GH1-Myb/GH1-Myb-related. The above pattern is generally conserved in angiosperm plants, as shown by a maximum-likelihood phylogenetic tree of GH1-containing proteins from a broad range of plant species (Supplemental Fig. S1). The split into typical H1s and GH1-HMGA/GH1-HMGA-related preceded the separation of the GH1-Myb/GH1-Myb-related subgroup. The rapid diversification of the latter compared with the H1s suggests that it was not initially subjected to strong purifying selection but might have been important for the ongoing adaptive evolution of plants. Perhaps this could be the reason that genes encoding Arabidopsis GH1-containing proteins other than H1s show differential expression patterns in different tissues and developmental stages (Supplemental Fig. S3; Schmidt et al., 2011). Below, we discuss the properties of the three subgroups in more detail.
Figure 1.
Maximum-likelihood phylogenetic tree and domain architecture of Arabidopsis GH1-containing proteins. Protein sequences were aligned with the local pair iterative algorithm implemented in Mafft (Yamada et al., 2016). Conserved columns from each multiple sequence alignment were selected manually. The phylogenetic analysis was performed with PhyML (Guindon et al., 2005), with the JTT model of amino acid substitutions and three random starting trees. Approximate likelihood ratio test SH-like (Shimodaira-Hasegawa-like) branch supports above 50% are shown. The tree was rooted using GH1-Myb as an internal sister outgroup for both GH1-HMGA and histone H1 clades. The tree image was prepared with iTol (Letunic and Bork, 2011). Domain architecture analysis was carried out using the SMART (Letunic et al., 2015) and GeneSilico (Kurowski and Bujnicki, 2003) Web servers and Meta-BASIC (Ginalski et al., 2004).
H1s
We have argued previously that the formal criteria that define a typical linker histone (i.e. a protein with a GH1 domain flanked by two unstructured and highly basic tails) are fulfilled by the products of only three Arabidopsis genes, designated H1.1, H1.2, and H1.3 (Wierzbicki and Jerzmanowski, 2005). As shown in Figure 1, the subgroup of Arabidopsis H1s consists exclusively of this trio of H1s, none of which has any recognizable domain except GH1. Consistent with earlier analyses of phylogenetic relationships among known plant linker histones (Jerzmanowski et al., 2000; Rutowicz et al., 2015), this subgroup contains a representative (H1.3) of a distinct branch of stress-inducible H1 variants (Ascenzi and Gantt, 1997, 1999; Scippa et al., 2000, 2004; Przewloka et al., 2002; Jerzmanowski, 2007). Previously, we demonstrated that this branch separated from the main H1 variants roughly 140 million years ago, which coincided with the appearance of angiosperm plants on Earth (Rutowicz et al., 2015). There are no orthologs of stress-inducible H1 variants in sequenced species representing green algae, bryophytes, lycophytes, and conifers (gymnosperms; analyzed in Supplemental Fig. S1). Importantly, only members of the H1 subgroup possess the characteristic regions of strong positive charge in all C-terminal and most N-terminal domains (Supplemental Fig. S2), beginning immediately adjacent to GH1. It should be noted that the regions of the N-terminal domains of H1.1 and H1.2 most distant from GH1 contain a negatively charged fragment that is targeted by posttranslational modification of phosphorylation, which further increases its negative charge (Kotliński et al., 2016). Thus, among Arabidopsis GH1-containing proteins, the pattern of charge distribution in the N- and C-terminal domains of H1s appears to be as distinctive a feature as the phylogenetic positions of their GH1s.
GH1-HMGA/GH1-HMGA-Related Versus Putative True Arabidopsis HMGA Proteins
In animals, HMGA proteins are distinguished by multiple AT-hook DNA-binding motifs: conserved nine-amino acid peptides capable of strong binding to 6-bp or longer AT-rich stretches of DNA via the minor groove. Except for an acidic C-terminal region, these proteins do not have any other recognized domains. In contrast, proteins currently defined in the literature as plant HMGA members contain a typical GH1 domain in addition to AT-hook motifs. This arrangement is restricted to angiosperm plants (Supplemental Fig. S1), suggesting a relatively late occurrence of GH1-AT-hook fusion in the evolution of plants. Arabidopsis has three such proteins (GH1-HMGA1 to GH1-HMGA3), which possess four to six AT-hook motifs. All three were detected in our analysis of the nuclear proteome of an Arabidopsis T87 cell suspension culture (Supplemental Table S1; http://proteome.arabidopsis.pl). Interestingly, the Arabidopsis GH1-HMGA cluster also includes a protein with no AT-hook domains (AT5G08780.1, named GH1-HMGA-related4 in our proposed nomenclature). We were unable to detect this protein in our T87 nuclear proteome (Supplemental Table S1), but its transcript was present in an Arabidopsis transcriptome derived by RNA sequencing analysis (Supplemental Table S1). Its GH1 sequence places GH1-HMGA-related4 distantly from the rest of the Arabidopsis H1-HMGA subgroup. Comparison of the charged amino acid profiles of non-GH1 fragments of Arabidopsis GH1-containing proteins demonstrated that the CTDs of GH1-HMGA1 to GH1-HMGA3 have an island-like distribution of positively and negatively charged residues, with mostly the latter present in fragments directly adjacent to GH1 (Supplemental Fig. S2). The corresponding profile for GH1-HMGA-related4 is significantly different. Secondary structure predictions suggest a potentially novel domain that lacks sequence similarity to any other protein domain of known or unknown structure/function. Interestingly, similar sequences are present in proteins from other species of the order Brassicales, in which they also are accompanied by GH1. The phylogenetic tree of GH1s from model plant proteomes identifies a distinct cluster composed of Arabidopsis GH1-HMGA-related4 and similar proteins from other species. Importantly, according to the InterPro database (http://www.ebi.ac.uk/interpro/), some of the proteins from other species belonging to this cluster retained AT-hook motifs.
The fusion of genuine GH1 and multiple AT-hook motifs that occurred in angiosperm plants also can be found in phylogenetic groups outside the plant kingdom, such as in numerous fish species, in Trichoplax adhaerens, the only extant representative of the phylum Placozoa (a primitive group of multicellular animals), as well as in some yeast, nematode, and insect species. The fish and T. adhaerens genomes encode very large proteins (up to 2,900 amino acids) in which GH1 and AT-hook motifs cooccur with RING and PHD domains. The other mentioned organisms possess simpler proteins in which GH1 coexists exclusively with AT-hook motifs. The phylogenetic relationships among these extremely diverse organisms suggest that multiple evolutionary events have resulted in the cooccurrence of GH1 and AT-hook motifs within their proteins.
Surprisingly given the fundamental functions of HMGA proteins in animals, the functional significance of the GH1/multiple AT-hook motif fusion has never been studied, despite its being referred to in all the major literature concerning plant HMG proteins. Notably, in several prokaryotes in which either HMGA-like or histone H1 CTD-like domains are present in important hub proteins regulating critical cellular processes, these two domains were found to be functionally equivalent and could be interchanged without any phenotypic consequences. Moreover, even chimeras in which the AT-hook domain was substituted by the human histone H1 CTD or full-length human H1 functioned properly in prokaryotic hosts (García-Heras et al., 2009). Thus, Arabidopsis GH1-HMGA proteins may be considered as highly specialized derivatives of H1 in which the typical CTD of H1 has been replaced by HMGA. To try and verify such a possibility, we reexamined the long-held view that Arabidopsis is devoid of canonical HMGA proteins. Using the SMART tool (Schultz et al., 2000; http://smart.embl-heidelberg.de), we identified 48 Arabidopsis proteins containing AT-hook motifs, 23 of which, unlike typical HMGA members, contain only a single AT hook. Most of the identified proteins, including those of the H1-HMGA subgroup, contain additional domains. Only two proteins, the predicted products of the alternatively spliced At1g48610 gene, contain four AT-hook motifs and no other domain. At1g48610.1 encodes a relatively small protein (212 amino acids, about 21.6 kD) with a high pI (pI = 11.6), features typical for HMGA. At1g48610.2 (transcript retains the last intron) encodes a shorter protein with a pI of 11.4. The other putative proteins with the AT-hook motif are significantly larger, and their pI, unlike that of canonical HMGA, is below 10. Interestingly, a protein encoded by At1g48610 was detected in our analyses of the nuclear proteome of Arabidopsis T87 cells, with a score and peptide number similar to those of core and linker histones, which indicated a substantial concentration in nuclei (http://proteome.arabidopsis.pl). Moreover, and probably due to its high pI, it was copurified during the isolation of Arabidopsis linker histones by extraction with 4.5% PCA (perchloric acid) and cation-exchange chromatography (Kotliński et al., 2016).
In both analyses, the larger version of AT1G48610 had a higher number of peptides and a higher score than the smaller form (100% and 92% of sequence coverage, respectively). Using four different proteases (trypsin, ArgC, termolysin, and pepsin), we identified 516 peptides unique for AT1G48610.1 (i.e. matching the last 29 amino acids of this protein), including peptides spanning the exon-exon junction. However, we detected no peptides unique for the smaller AT1G48610.2 form (i.e. matching the last 14 amino acids that are different in this variant). Similarly, RNA sequencing analysis revealed multiple reads spanning the junction of the last two exons of the gene but only one low-quality read within the intron retained in AT1G48610.2. These data indicate that the larger version of the protein (AT1G48610.1) is the main product of this gene. According to the BAR Toronto database (Toufighi et al., 2005), the expression of At1g48610 is strongest in the central, rib, and peripheral zones of the shoot apical meristem, in pistil tissue primarily consisting of ovaries, and in phloem companion cells at the border of the meristematic and elongation zones of the root. This suggests that AT1G48610, which we believe to be a true Arabidopsis HMGA protein, is important in the differentiation of stem cells, a role highly reminiscent of that played by animal HMGA-type proteins (Ozturk et al., 2014). Interestingly, the At1g48610 locus in chromosome 1 is located next to that encoding the H1-HMGA2 protein.
GH1-Myb/GH1-Myb-Related
This subgroup comprises five proteins with an additional N-terminal Myb domain accompanied by a 17- to 18-amino acid-long Myb extension-like domain. They seem to be as evolutionarily old as H1s, as, in addition to angiosperms, they occur in representatives of green algae, bryophytes, lycophytes, and gymnosperms (Supplemental Fig. S1). They are known as Single Myb Histone (SMH) or Telomere Repeat Binding (TRB) proteins, and two of them, GH1-Myb-TRB1 and GH1-Myb-TRB2, were shown to bind Arabidopsis telomeric repeats in vitro through a Myb domain of the telobox (telomere motif AAACCCTAA) type (Marian et al., 2003; Schrumpfová et al., 2004). The demonstration of in vivo interactions of these proteins with Arabidopsis telomerase supports a suggestion that they are part of the greater plant telomeric interactome (Schrumpfová et al., 2014). However, a recent mapping by chromatin immunoprecipitation sequencing of the genome-wide distribution of TRB1:GFP revealed its presence in over 7,800 genomic loci. The majority of these loci contained telobox-related motifs located at the transcription start sites, with additional loci spreading across gene bodies as well as distal promoter regions. Moreover, it was shown by genome-wide expression (RNA sequencing) analysis that TRB1, by binding at these loci, plays the role of transcriptional regulator, which is independent of its role in telomere maintenance (Zhou et al., 2016). Given such widespread occurrence, it seems highly probable that, at least in some of the detected loci, TRB1, through its GH1 domain, competes for nucleosome binding with H1s.
Since GH1-Myb-TRB3 is very similar to GH1-Myb-TRB1 and GH1-Myb-TRB2 (all three locate on the same branch of the phylogenetic tree; Supplemental Fig. S1), it may perform the same function. GH1-Myb-TRB1 was identified in our proteomic analysis of Arabidopsis nuclei, while GH1-Myb-TRB2 and GH1-Myb-TRB3 were detected below the established threshold (Supplemental Table S1). Transcripts encoding GH1-Myb-TRB1 to GH1-Myb-TRB3 were all present in our RNA sequencing data. Two other GH1-Myb proteins, GH1-Myb4 and GH1-Myb5 (AT1G17520.1 and AT1G72740.1, respectively), are more distantly related to GH1-Myb-TRB1 to GH1-Myb-TRB3 (Supplemental Fig. S1). The three other proteins of this subgroup (GH1-Myb-related6 to GH1-Myb-related8) lack the Myb domain, although the transcript of one them (AT1G54260.1) contains a Myb-coding sequence in front of the start codon, suggesting the loss of this domain during evolution. AT1G54260.1 also contains a strongly diverged and truncated GH1 domain at the C-terminal side of its regular GH1 domain. According to secondary structure predictions, the two other proteins lacking the Myb domain (AT1G54230 and AT1G54240) have α-helical regions within their CTDs. Interestingly, all three proteins lacking Myb are encoded by neighboring genes on chromosome 1. The N- and C-terminal domains of all proteins from the GH1-Myb/GH1-Myb-related subgroup are mostly negatively charged.
A Rationale for the Proposed New Nomenclature of Arabidopsis GH1-Containing Proteins
At first glance, the evolutionary diversification of H1s into well-distinguished and conserved subtypes seems to be less pronounced in angiosperm plants than in animals, particularly vertebrates. The most distinct structural and functional diversification of plant H1s coincided with the appearance of angiosperms (approximately 140 million years ago) and resulted in two major subtypes that have been maintained ever since: the main and stress-inducible H1s. Regarding H1s, the case of Arabidopsis shows that two main variants and a single stress-inducible variant are sufficient to support the basic processes of growth and development in a typical flowering plant. While this does not rule out the functional significance of more subtle variation within these two major subtypes observed in systematically distant families and species, proof of such significance has yet to be provided. The above notwithstanding, the impression of a seemingly limited diversification of H1s during the evolution of plants may be misleading and result from biased classification rules. These rules were adopted from studies on typical animal H1s and do not take into account the fundamentally different life strategies and vastly different selection pressures shaping major chromatin structural proteins in plants and animals during their long histories of separate evolution. The GH1-HMGA/GH1-HMGA-related and GH1-Myb/GH1-Myb-related subgroups could be the end result of such specific selection pressures in the plant kingdom. The concept that proteins of these two subgroups represent highly diverged and specialized derivatives of plant H1 that use GH1 as a common motif for targeting nucleosomes is supported by the conserved phylogenetic relationships among plant GH1-containing proteins, a recently demonstrated widespread occurrence of GH1-Myb-TRB1 in chromatin, and its likely involvement in transcriptional regulation, as well as by the identification of a candidate for a true Arabidopsis HMGA protein that does not contain a GH1 domain. This concept is by no means equivalent to suggesting that all plant GH1-containing proteins are bona fide H1 variants, in a sense ascribed to this subcategory in animal studies. Its main purpose is to draw attention to the fact that, in plants, the competition-based removal of H1 from chromatin may be dependent on a more diversified and specialized group of competitors than in animals, suggesting novel plant-specific mechanisms of chromatin regulation.
Therefore, we propose a unified nomenclature for plant GH1-containing proteins built simply on their GH1-based phylogenetic relationships, as shown in Figure 1. We further propose to distinguish proteins possessing two characteristic domains (GH1-HMGA and GH1-Myb) and proteins belonging to the same subgroups due to the phylogenetic position of their GH1 but lacking the second characteristic domain, HMGA or Myb. We name these latter proteins GH1-HMGA-related and GH1-Myb-related, respectively (they are marked by lighter color in Supplemental Fig. S1). It is important to remember that proteins of these two types from other species still retain their AT-hook motifs and Myb domains. Since the GH1-Myb-TRB1 and GH1-Myb-TRB2 proteins have been experimentally confirmed to bind telomere repeats and, therefore, were named TRB1 and TRB2, we propose to retain this functional reference in their names (as GH1-Myb-TRB) for the sake of clarity and tradition. The same applies to GH1-Myb-TRB3, a very similar protein that has been described previously as TRB3. With regard to GH1-Myb4 and GH1-Myb5, which also are described as TRB proteins in many databases, we suggest removing the designation TRB from their names. In the Arabidopsis GH1 evolutionary tree, both of these proteins group in a clade separate from that of TRB1 to TRB3, suggesting a greater evolutionary distance. Moreover, and unlike GH1-Myb-TRB1 to GH1-Myb-TRB3, they both contain a Myb extension-like sequence different from GH1-Myb-TRB1 to GH1-Myb-TRB3, so their binding preferences may be different. We also have indicated (Supplemental Table S1; Supplemental Fig. S1, parentheses) the former names of GH1-Myb proteins as SMH that were used in the discontinued ChromDB and in maize (Zea mays) genomic databases. Importantly, our inspection in SMART/UniProt of the domain structures of all proteins included in the tree in Supplemental Figure S1 revealed some singularities. In Medicago truncatula, a GH1-Myb protein has an additional RNA-recognition motif. Another GH1-Myb of this species has a strongly changed GH1 domain. Both Brassica rapa and Oryza sativa have a GH1-Myb protein carrying an additional domain, and maize contains a GH1-HMGA protein with an S/T kinase domain. Moreover, in the maize H1 group, there is a protein with two AT-hook motifs (indicative that such fusions are not unusual in plants). While exception proves the rule, it cannot be excluded that at least some of the above singularities resulted from errors in genome assemblies or gene models.
We believe that the proposed phylogeny- and structure-supported system of classification, apart from practical convenience, will foster novel approaches in studies on the functional roles of GH1-containing proteins in plants.
MATERIALS AND METHODS
Database Screen
All proteins from TAIR (http://arabidopsis.org) and protein records from Arabidopsis (Arabidopsis thaliana) deposited in the NCBInr (https://www.ncbi.nlm.nih.gov/) and UniProt (http://www.uniprot.org/) databases were searched with the use of BLAST (Altschul et al., 1990) for proteins containing a GH1 domain. Sequences of GH1 from all 15 Arabidopsis GH1-containing proteins were used as queries. All records found are included in Table I and Supplemental Table S1 (Fucile et al. 2011). Additionally, the full genomic sequence from TAIR repository was translated in six reading frames and searched by position-specific iterated BLAST (Altschul et al., 1997). All 15 GH1 sequences from known proteins were used as queries. We have not found any new GH1-containing proteins in Arabidopsis.
Domain Architecture
Domain architecture analysis was carried out for all Arabidopsis proteins containing a GH1 domain using Meta-BASIC (Ginalski et al., 2004) as well as SMART (Letunic et al., 2015) and GeneSilico (Kurowski and Bujnicki, 2003) Web servers. The regions with no detectable homology to known protein domains, yet with conserved sequence and predicted secondary structures (with PSIPRED; Jones, 1999), also have been denoted as potential new domains.
For proteins assigned previously to the GH1-Myb subfamily yet lacking the Myb domain, nucleotide upstream/downstream sequences of coded genes were verified using both manual translations and data from TAIR gene model and exon confidence ranking system (https://www.arabidopsis.org/download_files/Genes/TAIR10_genome_release/TAIR10_gene_confidence_ranking/DOCUMENTATION_TAIR_Gene_Confidence.pdf). The truncated GH1 domain detected in AT1G54260 was verified in a similar manner.
Moving-Sum Plot
A moving-sum plot of net charge was generated for both N- and C-terminal regions (with respect to the GH1 domain) of all Arabidopsis GH1-containing proteins. The net charge was summed in a 20-amino acid sliding window along N- and C-terminal regions, starting from the GH1 domain. For each region, the percentages of both positively (K, R) and negatively (D, E) charged residues, total charge, and theoretical pI (calculated with http://web.expasy.org/compute_pi) also were calculated.
Phylogenetic Analyses
Protein sequences for model plants were collected via phmmer (Finn et al., 2011), available from the Ensembl Plants Web site (Kersey et al., 2014). The Ensembl database was chosen to ensure data quality, limiting the data set to well-studied organisms with possibly complete proteomes. This data set enables observations of specific subfamily expansions (due to consecutive duplications) in some angiosperms from Brassicaceae and Fabaceae. For better taxon sampling, the following representatives of missing major taxon groups were added: Auxenochlorella protothecoides, Coccomyxa subellipsoidea, Marchantia polymorpha, Picea sitchensis, Pinus taeda (from UniProt), and Klebsormidium flaccidum (from NCBI genomes).
Sequence searches were performed using H1.2, GH1-HMGA2, GH1-Myb-TRB1, and TRB1 from Arabidopsis as queries. All hits were mapped on UniProt identifiers (http://www.uniprot.org), except for Physcomitrella patens (which lacks UniProt identifiers for two out of nine analyzed sequences). Subsequently, representative plants were chosen with emphasis on Brassicaceae (three taxa) and including all basal plant model organisms present in the aforementioned database (for a list of identifiers and names, see Supplemental Table S3). Incomplete truncated sequences were discarded. Phylogenetic trees were inferred both for Arabidopsis GH1 proteins (Fig. 1) and for 282 representative plant sequences (Supplemental Fig. S1).
Sequences of all GH1-containing proteins used for phylogenetic comparison were screened with SMART (Schultz et al., 2000; Letunic et al., 2015) for the presence of any additional domains or loss of domains (other than GH1). The results are included in Supplemental Figure S1.
Accession Numbers
Sequence data from this article can be found are provided in tables, figures and Supplemental Data.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Maximum-likelihood phylogenetic tree of GH1-containing proteins from selected plants.
Supplemental Figure S2. Moving-sum plot of net charge for N- and C-terminal domains of all Arabidopsis GH1-containing proteins.
Supplemental Figure S3. Relative expression levels of GH1-containing protein-coding genes in Arabidopsis across 74 tissue- or cell-specific microarrays.
Supplemental Table S1. Accession numbers and descriptions of Arabidopsis proteins containing a GH1 domain from different databases (TAIR10, UniProt, NCBInr, and ChromDB).
Supplemental Table S2. List of articles referring to the role of plant linker histones.
Supplemental Table S3. List of GH1-containing protein identifiers in selected model plants.
Supplemental Methods. Supplemental materials and methods.
Supplementary Material
Acknowledgments
We thank Fred Berger, Franziska Turck, Fredy Barneche, J. Mark Cock, Lars Hennig, Motoaki Seki, Anna Amtmann, and Doris Wagner for comments and fruitful discussions.
Glossary
- CTD
C-terminal domain
Footnotes
This work was supported by the European Cooperation in Science and Technology and the Ministry of Science and Higher Education (grant no. MNiSW 212/N–COST/2008/0 to A.J. and M.K.), the Foundation for Polish Science (TEAM to K.G.) and the National Science Centre (grant nos. 2011/02/A/NZ2/00014 and 2014/15/B/NZ1/03357 to K.G.), the National Science Centre (grant no. 2012/07/D/NZ2/04286 to A.M.), the Ministry of Science and Higher Education (scholarship for outstanding young researchers to A.M.), and the Swiss National Science Foundation (grant no. 31003A_149974/1 to C.B.).
Articles can be viewed without a subscription.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascenzi R, Gantt JS (1997) A drought-stress-inducible histone gene in Arabidopsis thaliana is a member of a distinct class of plant linker histone variants. Plant Mol Biol 34: 629–641 [DOI] [PubMed] [Google Scholar]
- Ascenzi R, Gantt JS (1999) Molecular genetic analysis of the drought-inducible linker histone variant in Arabidopsis thaliana. Plant Mol Biol 41: 159–169 [DOI] [PubMed] [Google Scholar]
- Brown DT, Izard T, Misteli T (2006) Mapping the interaction surface of linker histone H1(0) with the nucleosome of native chromatin in vivo. Nat Struct Mol Biol 13: 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 39: W29–W37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile G, Di Biase D, Nahal H, La G, Khodabandeh S, Chen Y, Easley K, Christendat D, Kelley L, Provart NJ (2011) ePlant and the 3D data display initiative: integrative systems biology on the world wide web. PLoS ONE 6: e15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Heras F, Padmanabhan S, Murillo FJ, Elías-Arnanz M (2009) Functional equivalence of HMGA- and histone H1-like domains in a bacterial transcriptional factor. Proc Natl Acad Sci USA 106: 13546–13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginalski K, von Grotthuss M, Grishin NV, Rychlewski L (2004) Detecting distant homology with Meta-BASIC. Nucleic Acids Res 32: W576–W581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, Gascuel O (2005) PHYML Online: a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res 33: W557–W559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JC, Lu X, Ross ED, Woody RW (2006) Intrinsic protein disorder, amino acid composition, and histone terminal domains. J Biol Chem 281: 1853–1856 [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Lever MA, Crawford E, Th’ng JPH (2004) The C-terminal domain is the primary determinant of histone H1 binding to chromatin in vivo. J Biol Chem 279: 20028–20034 [DOI] [PubMed] [Google Scholar]
- Izzo A, Kamieniarz K, Schneider R (2008) The histone H1 family: specific members, specific functions? Biol Chem 389: 333–343 [DOI] [PubMed] [Google Scholar]
- Jerzmanowski A. (2004) The linker histones. In Chromatin Structure and Dynamics: State-of-the-Art. New Comprehensive Biochemistry, Vol 39 Elsevier, Amsterdam, The Netherlands: pp 75–102 [Google Scholar]
- Jerzmanowski A. (2007) SWI/SNF chromatin remodeling and linker histones in plants. Biochim Biophys Acta 1769: 330–345 [DOI] [PubMed] [Google Scholar]
- Jerzmanowski A, Przewłoka M, Grasser KD (2000) Linker histones and HMG1 proteins of higher plants. Plant Biol 2: 586–597 [Google Scholar]
- Jones DT. (1999) Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 292: 195–202 [DOI] [PubMed] [Google Scholar]
- Kersey PJ, Allen JE, Christensen M, Davis P, Falin LJ, Grabmueller C, Hughes DST, Humphrey J, Kerhornou A, Khobova J, et al. (2014) Ensembl Genomes 2013: scaling up access to genome-wide data. Nucleic Acids Res 42: D546–D552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotliński M, Rutowicz K, Kniżewski Ł, Palusiński A, Olędzki J, Fogtman A, Rubel T, Koblowska M, Dadlez M, Ginalski K, et al. (2016) Histone H1 variants in Arabidopsis are subject to numerous post-translational modifications, both conserved and previously unknown in histones, suggesting complex functions of H1 in plants. PLoS ONE 11: e0147908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowski MA, Bujnicki JM (2003) GeneSilico protein structure prediction meta-server. Nucleic Acids Res 31: 3305–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P (2011) Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res 39: W475–W478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P (2015) SMART: recent updates, new developments and status in 2015. Nucleic Acids Res 43: D257–D260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian CO, Bordoli SJ, Goltz M, Santarella RA, Jackson LP, Danilevskaya O, Beckstette M, Meeley R, Bass HW (2003) The maize Single myb histone 1 gene, Smh1, belongs to a novel gene family and encodes a protein that binds telomere DNA repeats in vitro. Plant Physiol 133: 1336–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryant SJ, Lu X, Hansen JC (2010) Multifunctionality of the linker histones: an emerging role for protein-protein interactions. Cell Res 20: 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Over RS, Michaels SD (2014) Open and closed: the roles of linker histones in plants and animals. Mol Plant 7: 481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk N, Singh I, Mehta A, Braun T, Barreto G (2014) HMGA proteins as modulators of chromatin structure during transcriptional activation. Front Cell Dev Biol 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prymakowska-Bosak M, Przewłoka MR, Iwkiewicz J, Egierszdorff S, Kuraś M, Chaubet N, Gigot C, Spiker S, Jerzmanowski A (1996) Histone H1 overexpressed to high level in tobacco affects certain developmental programs but has limited effect on basal cellular functions. Proc Natl Acad Sci USA 93: 10250–10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewloka MR, Wierzbicki AT, Slusarczyk J, Kuraś M, Grasser KD, Stemmer C, Jerzmanowski A (2002) The “drought-inducible” histone H1s of tobacco play no role in male sterility linked to alterations in H1 variants. Planta 215: 371–379 [DOI] [PubMed] [Google Scholar]
- Rutowicz K, Puzio M, Halibart-Puzio J, Lirski M, Kotliński M, Kroteń MA, Knizewski L, Lange B, Muszewska A, Śniegowska-Świerk K, et al. (2015) A specialized histone H1 variant is required for adaptive responses to complex abiotic stress and related DNA methylation in Arabidopsis. Plant Physiol 169: 2080–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho M, Diani E, Beato M, Jordan A (2008) Depletion of human histone H1 variants uncovers specific roles in gene expression and cell growth. PLoS Genet 4: e1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Wuest SE, Vijverberg K, Baroux C, Kleen D, Grossniklaus U (2011) Transcriptome analysis of the Arabidopsis megaspore mother cell uncovers the importance of RNA helicases for plant germline development. PLoS Biol 9: e1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrumpfová P, Kuchar M, Miková G, Skrísovská L, Kubicárová T, Fajkus J (2004) Characterization of two Arabidopsis thaliana myb-like proteins showing affinity to telomeric DNA sequence. Genome 47: 316–324 [DOI] [PubMed] [Google Scholar]
- Schrumpfová PP, Vychodilová I, Dvořáčková M, Majerská J, Dokládal L, Schořová S, Fajkus J (2014) Telomere repeat binding proteins are functional components of Arabidopsis telomeres and interact with telomerase. Plant J 77: 770–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Copley RR, Doerks T, Ponting CP, Bork P (2000) SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res 28: 231–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scippa GS, Di Michele M, Onelli E, Patrignani G, Chiatante D, Bray EA (2004) The histone-like protein H1-S and the response of tomato leaves to water deficit. J Exp Bot 55: 99–109 [DOI] [PubMed] [Google Scholar]
- Scippa GS, Griffiths A, Chiatante D, Bray EA (2000) The H1 histone variant of tomato, H1-S, is targeted to the nucleus and accumulates in chromatin in response to water-deficit stress. Planta 211: 173–181 [DOI] [PubMed] [Google Scholar]
- She W, Grimanelli D, Rutowicz K, Whitehead MWJ, Puzio M, Kotlinski M, Jerzmanowski A, Baroux C (2013) Chromatin reprogramming during the somatic-to-reproductive cell fate transition in plants. Development 140: 4008–4019 [DOI] [PubMed] [Google Scholar]
- Stasevich TJ, Mueller F, Brown DT, McNally JG (2010) Dissecting the binding mechanism of the linker histone in live cells: an integrated FRAP analysis. EMBO J 29: 1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SH, Goutte-Gattat D, Becker N, Meyer S, Shukla MS, Hayes JJ, Everaers R, Angelov D, Bednar J, Dimitrov S (2010) Single-base resolution mapping of H1-nucleosome interactions and 3D organization of the nucleosome. Proc Natl Acad Sci USA 107: 9620–9625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufighi K, Brady SM, Austin R, Ly E, Provart NJ (2005) The Botany Array Resource: e-northerns, expression angling, and promoter analyses. Plant J 43: 153–163 [DOI] [PubMed] [Google Scholar]
- Wierzbicki AT, Jerzmanowski A (2005) Suppression of histone H1 genes in Arabidopsis results in heritable developmental defects and stochastic changes in DNA methylation. Genetics 169: 997–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski JR, Zougman A, Kruger S, Mann M (2007) Mass spectrometric mapping of linker histone H1 variants reveals multiple acetylations, methylations, and phosphorylation as well as differences between cell culture and tissue. Mol Cell Proteomics 6: 72–87 [DOI] [PubMed] [Google Scholar]
- Woodcock CL, Skoultchi AI, Fan Y (2006) Role of linker histone in chromatin structure and function: H1 stoichiometry and nucleosome repeat length. Chromosome Res 14: 17–25 [DOI] [PubMed] [Google Scholar]
- Yamada KD, Tomii K, Katoh K (2016) Application of the MAFFT sequence alignment program to large data: reexamination of the usefulness of chained guide trees. Bioinformatics 32: 3246–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A, Kim MY, Hsieh PH, Coleman-Derr D, Eshed-Williams L, Thao K, Harmer SL, Zilberman D (2013) The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell 153: 193–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BR, Feng H, Kato H, Dai L, Yang Y, Zhou Y, Bai Y (2013) Structural insights into the histone H1-nucleosome complex. Proc Natl Acad Sci USA 110: 19390–19395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Hartwig B, James GV, Schneeberger K, Turck F (2016) Complementary activities of TELOMERE REPEAT BINDING proteins and polycomb group complexes in transcriptional regulation of target genes. Plant Cell 28: 87–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YB, Gerchman SE, Ramakrishnan V, Travers A, Muyldermans S (1998) Position and orientation of the globular domain of linker histone H5 on the nucleosome. Nature 395: 402–405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.