Abstract
For Siewert type II adenocarcinoma of the esophagogastric junction (AEJ), the optimal surgical approach and extent of lymph nodes dissection remain controversial. Immunohistochemistry (IHC) has been reported to be available for identifying lymph node micrometastasis (LNMM) in patients with AEJ. This was a prospective case series of patients who underwent R0 resection and lower mediastinal lymphadenectomy from January 2010 to June 2015 in Fujian Medical University Union Hospital for Siewert type II AEJ. The outcomes were analyzed retrospectively. A total of 1325 lymph nodes were collected from 49 patients, grouped into 3 groups: lower mediastinal, paracardial, and abdominal. The former 2 groups were examined by monoclonal antibodies against Ber-Ep4 and CD44v6. The incidence of LNMM in mediastinal group was 37% (18/49) for Ber-Ep4 and 33% (16/49) for CD44v6. While in routine histological diagnosis, the number of patients with the positive lymph nodes was 7 (14%). When combining IHC with histopathology (HE) staining, the incidence of positive mediastinal lymph nodes was increased to 24%, with a total number of 37 lymph nodes from 28 patients (57%). Micrometastases indicated by Ber-Ep4 and CD44v6 were associated with the depth of tumor invasion (P = 0.020 and 0.037, respectively), histopathological nodal status (P = 0.024 and 0.01, respectively), and Lauren classification (P = 0.038 and, respectively). Expression of CD44v6 and Ber-Ep4 was positively correlated (r = 0.643, P < 0.001). The 3- and 5-year survival rates for all patients were 66% and 50%, respectively. The patients with LNMM had a lower 3-year survival rate of 51%, compared to 80% from no LNMM group; 5-year survival rate was also lower in LNMM group, which is 29% versus 68% (P = 0.006) in the no LNMM group. Patients with positive Ber-Ep4 cells had a lower survival, but not statistically significant (P = 0.058). CD44v6-positive group had a significantly reduced survival (P < 0.001). In patients group with negative lower mediastinal lymph nodes, patients without LNMM obtained a significant survival benefit (P = 0.021). Our study demonstrated that routine test for LNMM is necessary for patients with negative lymph nodes. As a positive prognostic factor, thorough lower mediastinal lymphadenectomy in an invasive approach should be considered when necessary. Ber-Ep4 and CD44v6 were shown to be great markers for detecting LNMM.
Keywords: adenocarcinoma of the esophagogastric junction, Ber-Ep4, CD44v6, lymph node micrometastasis
1. Introduction
In recent decades, the incidence of distal gastric adenocarcinoma has steadily decreased, whereas the rate of adenocarcinoma of the esophagogastric junction (AEJ) has been increasing rapidly both in Western and Eastern countries.[1,2] The Siewert classification defines AEJ into 3 types according to the center of the main tumor mass regarding the esophagogastric junction (EGJ).[3] This classification is now accepted and used worldwide. Siewert type I AEJ, distal esophageal adenocarcinoma, is located 1 to 5 cm above EGJ; Siewert type II AEJ, the true carcinoma of cardia, is located 1 cm oral to 2 cm aboral of the EGJ; and Siewert type III AEJ, subcardial gastric carcinoma, is 2 to 5 cm below the EGJ.
Radical surgery of tumor with lymph node dissection is now accepted for the treatment of AEJ. R0 resection is thought to be a dependent prognostic factor in the patients with AEJ.[4] However, regardless of all the alternative treatments, the 5-year survival rate is still not satisfactory.[4,5] For the Siewert types I and III AEJ, the surgical strategies are well elucidated.[6–8] But the optimal surgical approach and extent of lymph nodes dissection remain controversial in Siewert type II AEJ.
As we know, metastasis to lymph nodes is of great significance in esophageal and gastric tumors, especially in mediastinal tumors.[9–11] Even the patients with negative lymph node metastases have high chance of recurrences and metastases, mainly contributed by lymph node micrometastasis (LNMM), which was hard to detect before. With the recent advances in molecular diagnostic tools, LNMM can be identified more easily. These tools have been used widely in breast, gastric, and colon cancers.[12–14] Several researchers have demonstrated that LNMM might be a key prognostic factor for the survival rate in gastrointestinal cancer by the use of monoclonal antibodies.[15,16] Therefore, we use Ber-Ep4 antibody joint with CD44v6 for detecting the mediastinal LNMM to estimate the optimal extent of lymph node dissection.
2. Materials and methods
2.1. Patients
From January 2010 to June 2015, we collected a prospective cohort with the patients of the Department of Thoracic Surgery, in Fujian Medical University Union Hospital. The inclusion criteria were confirmed AEJ before operation, received R0 resection and lower mediastinal lymph node dissection, and validated Siewert type II after operation. The exclusion criteria were multiple lesions, other malignant tumor accompanied, and chemotherapy and/or radiotherapy before operation.
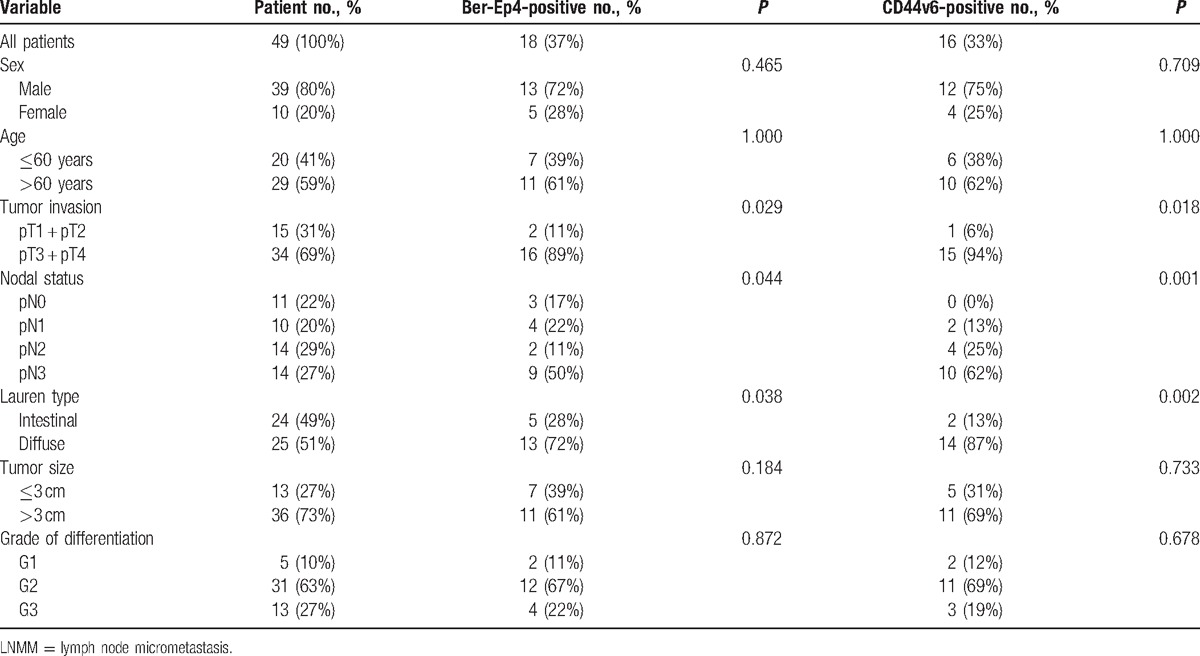
A total of 49 patients with pathologically confirmed Siewert type II AEJ were included in this study. Data on gender, sex, pTN-classification, tumor size, grade of differentiation, and Lauren classification are shown at Table 1. Median age was 65 years (43–80 years). There were 29 out of 49 patients (59%) aged more than 60 years.
Table 1.
Patients’ clinicopathological characteristics, and the relationships between lower mediastinal LNMM with clinicopathological parameters.
The ethics committee of authors’ university and hospital had approved this study, and patients’ consent was obtained before research.
2.2. Immunohistochemistry
LNMM is defined as tumor cells or cell cluster from 0.2 to 2.0 mm in diameter by the criteria of the tumor–node–metastasis classification established by the International Union Against Cancer.[17]
Epithelial makers are commonly used to indentify LNMM in IHC. According to previously published reports, Ber-Ep4 and CD44 variant 6 (CD44v6) antibodies are often used for diagnostic IHC.[16,18–20]
Three consecutive slices (4 μm thick) cut from the formalin-fixed, paraffin-embedded lymph node specimens were tested in IHC, using mouse antihuman Ber-Ep4 and CD44v6 monoclonal antibodies (Fuzhou Maxin Biotechnology Development, Fuzhou, China). The detection of LNMM was visualized by using diaminobenzidine chromogenic enzyme. Negative controls were acquired by omitting the primary antibodies. AEJ patients diagnosed with metastases on routine examination were used as positive control. Ber-Ep4-positive and CD44v6-positive staining appeared as brown/yellow coloration in cell membrane and/or cytoplasm (Fig. 1). Any tumor cells, single or in groups, detected by IHC were considered to be positive and confirmed in a high-power lens (×400). All the slides were assessed in a blinded fashion by 2 pathologists independently. Both observers obtained identical results in 95% of the slides, the remaining slides were reassessed before an accordant decision was made.
Figure 1.
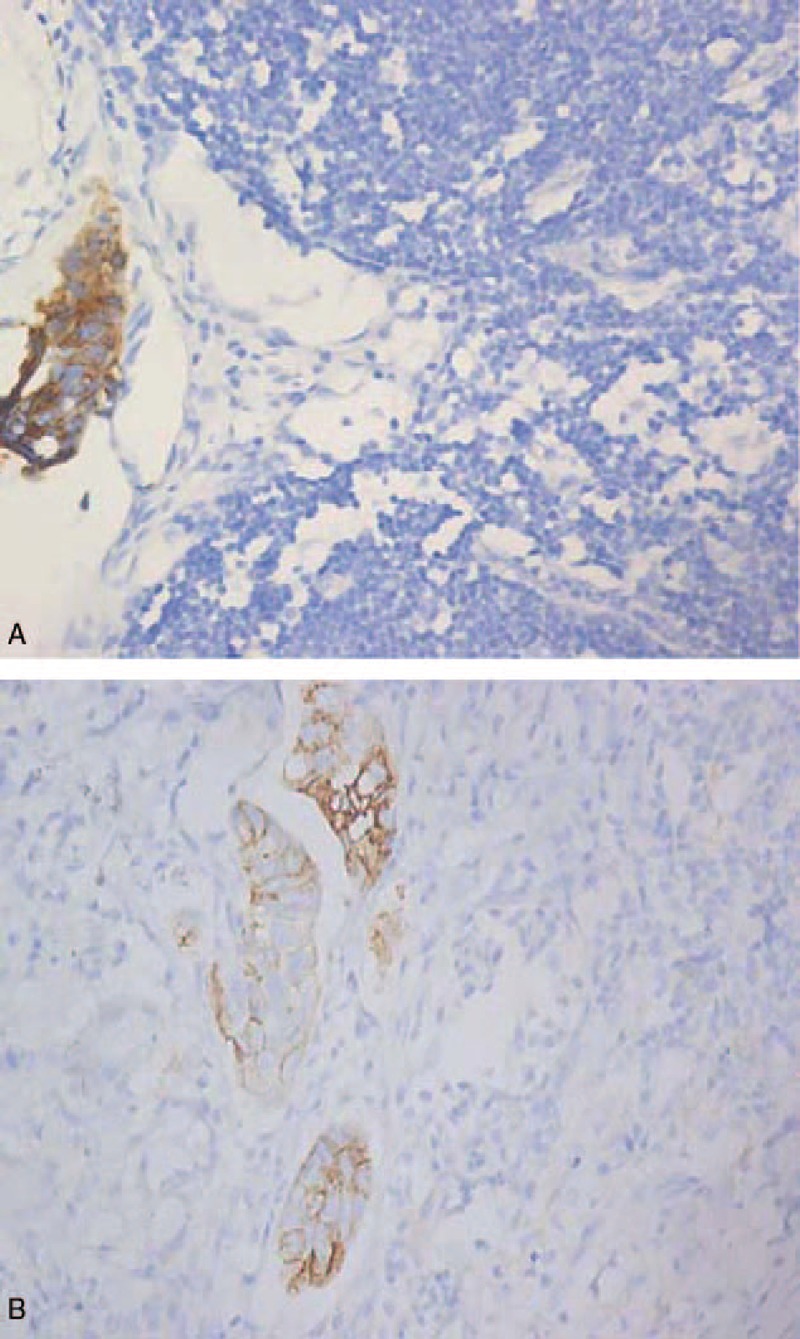
Lymph node slices from patients with Siewert type II adenocarcinoma of the esophagogastric junction show positive lymph node micrometastasis for (A) Ber-Ep4or (B) CD44v6 (magnification ×400).
2.3. Statistics
All data analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBMCorp., Armonk, NY). The relationships between lymph node micrometastasis and clinicopathological parameters were observed using the χ2 test. The correlation of expression of CD44v6 and Ber-Ep4 was calculated by Spearman rank correlation analysis. Survival rate was evaluated using Kaplan–Meier survival analysis and the log-rank test. The P value <0.05 was considered statistically significant.
3. Results
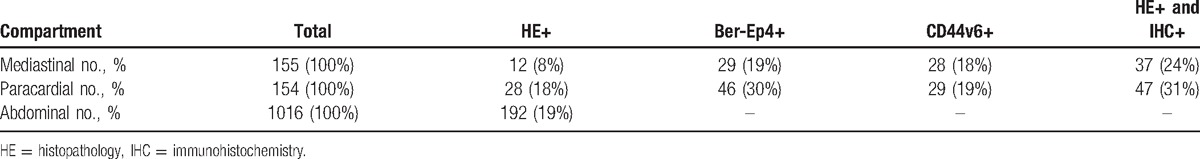
3.1. Clinicopathological characteristics
Forty-nine patients were enrolled in this study. Fifteen patients (31%) had pT1 + pT2 and 34 patients (69%) had pT3 + pT4. A total of 38 patients had regional lymph node metastasis in routine HE regardless of T stage (pN+, 76%), while 11 patients (22%) were pN0. A total of 13 patients (27%) had poorly differentiated tumors according to the grading. A total of 31 (63%) and 5 patients (10%) had moderately and well differentiated tumors, respectively. It is shown in Table 1 that overall 1325 lymph nodes were collected with 155 from lower mediastinum, 154 from paracardial region, and 1016 from abdomen (Table 2). All negative controls were defined by HE staining, Ber-Ep4, and CD44v6 staining. The 2 positive control specimens showing positive on routine examination were also positive for both Ber-Ep4 and CD44v6.
Table 2.
The number of positive lymph nodes of histopathology and IHC in the mediastinal, paracardial, and abdominal compartments.
3.2. Incidence of nodal Ber-Ep4+ and CD44v6+ cells
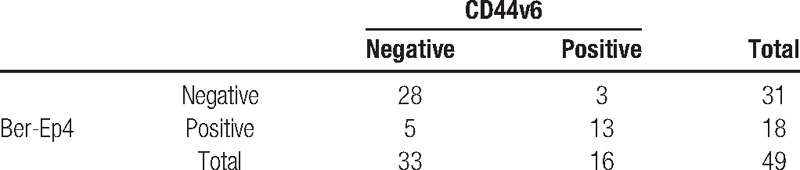
In HE, abdominal lymph node metastasis had the highest incidence (192-positive lymph nodes [19%]). Ber-Ep4+ and CD44v6+ cells were both observed in Siewert type II. HE staining showed 12 positive lymph nodes (8%) with mediastinal metastasis. However, IHC of Ber-Ep4 or CD44v6 increased the number into 29 (19%) and 28 (18%), respectively. Combining HE staining with IHC assessment, the number of positive lymph nodes was greatly increased (24%). In total, 309 lymph nodes were analyzed. Positive cells in the sinuses, the lymphoid interstitium, or in both locations were found in 75 lymph nodes (24%) by Ber-Ep4 and 57 (18%) by CD44v6. A total of 14 out of 42 patients (33%), who were classified to be “tumor-free” by conventional HE, were showed LNMM-positive indicated by the expression of Ber-Ep4 and CD44v6 in IHC. The relationship between LNMM and various clinicopathological features is shown in Table 1. The presence of Ber-Ep4+ or CD44v6+ cells in LNMM was significantly related to the depth of invasion (P = 0.029 and 0.018, respectively), nodal status (P = 0.044 and 0.001, respectively), and Lauren type (P = 0.038 and 0.002, respectively), but not to sex, age, degree of differentiation, or tumor size. Expression of CD44v6 and Ber-Ep4 was positively correlated (r = 0.643, P < 0.001, shown at Table 3).
Table 3.
The correlation of expression of CD44v6 and Ber-Ep4 (r = 0.643, P < 0.001).
3.3. Disease-specific survival
We lost 4 patients in follow-up. The median observation was 33 months (range, 1–73 months). The 3-year survival rate was 66% for all patients, 80% for LNMM patients, and 68% for no LNMM patients. The 5-year survival rate was 50% for all patients, 51% for LNMM patients, and 29% for no LNMM patients (log-rank test, P = 0.006; Fig. 2). Patients with positive Ber-Ep4 cells had a lower disease-specific survival, though it was not statistically significant (log-rank test, P = 0.058; Fig. 3). Patients with positive CD44v6 had a significantly reduced survival (P < 0.001; Fig. 4). We stratified patients of negative lower mediastinal lymph nodes from the positive group. In 42 patients with negative lower mediastinal lymph nodes, we observed a significant survival benefit in patients without LNMM (P = 0.021; Fig. 5).
Figure 2.
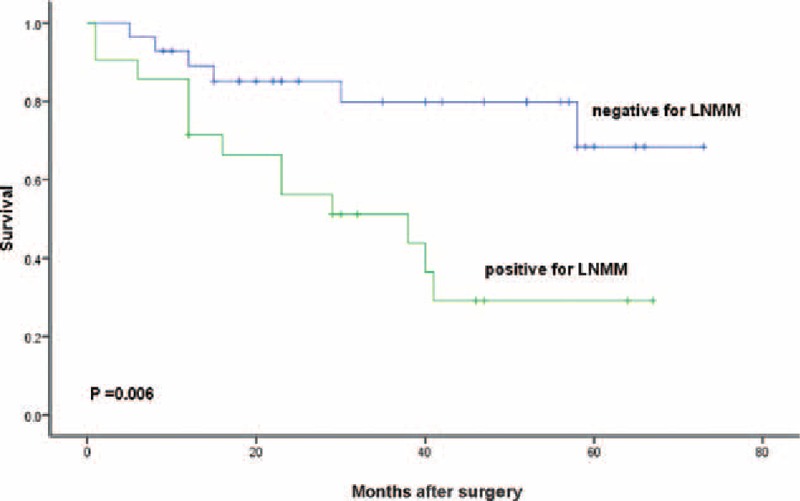
Survival of all patients with Siewert type II adenocarcinoma of the esophagogastric junction depending on positive or negative for lymph node micrometastasis. Kaplan–Meier, log-rank test.
Figure 3.
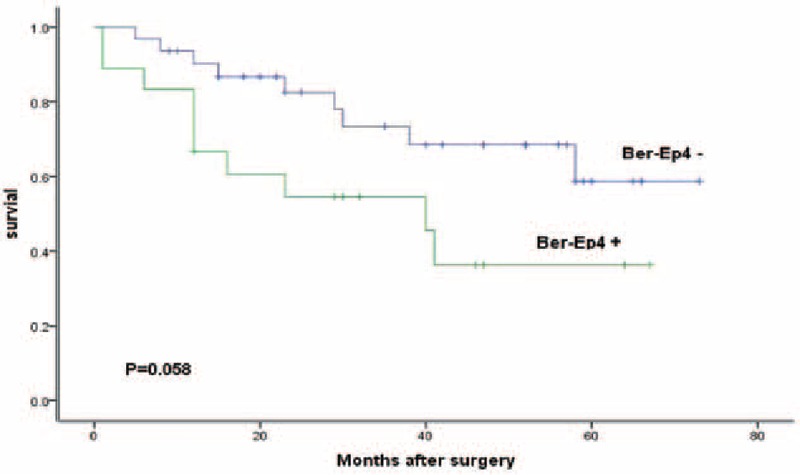
Survival of all patients depending on positive or negative for Ber-Ep4. Kaplan–Meier, log-rank test.
Figure 4.
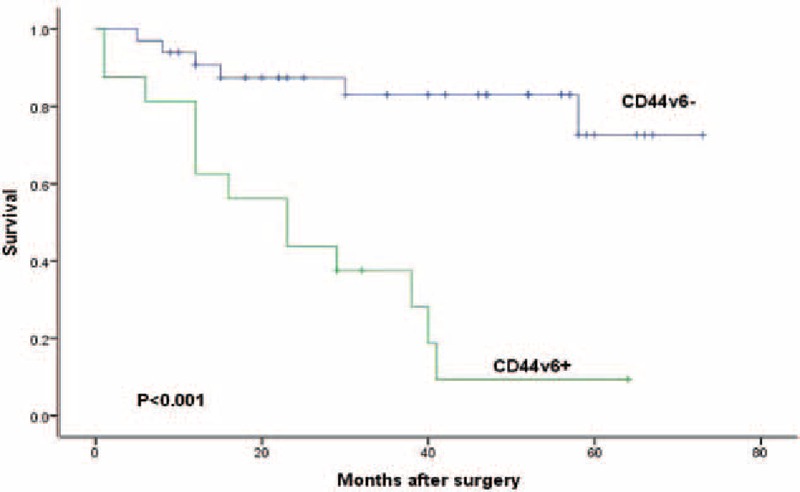
Survival of all patients depending on positive or negative for CD44v6. Kaplan–Meier, log-rank test.
Figure 5.
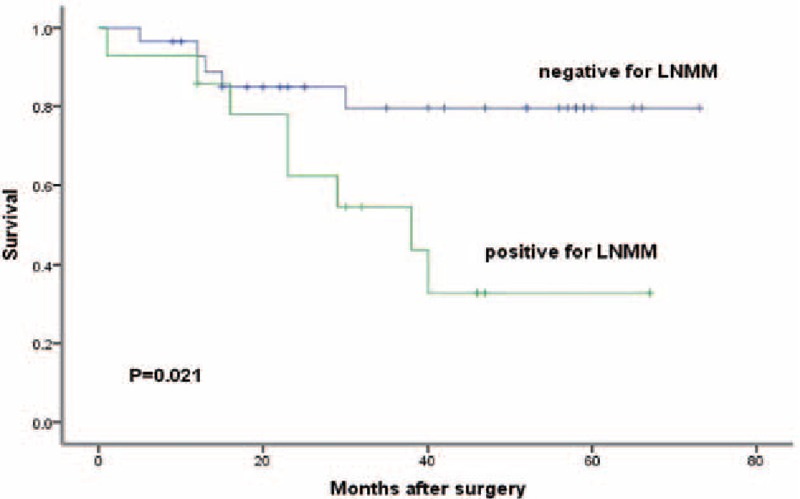
Kaplan–Meier survival curve of 42 patients with negative lower mediastinal lymph nodes in routine examination depending on positive or negative for lymph node micrometastasis. Kaplan–Meier, log-rank test.
4. Discussion
The incidence of AEJ has been increasing rapidly. Metastasis to lymph nodes plays a key role in prognosis. To date, the concept of LNMM, which can be detected by IHC easily, is widely accepted. Epithelial makers are commonly used. Ber-Ep4 is an antibody against 2 glycopolypeptides of 34 and 39 kD on the surface and the cytoplasm of all epithelial cells except the superficial layers of squamous epithelia, parietal cells, and hepatocytes. The antibody is not cross-reactive with mesenchymal cells, including the ones of lymphatic tissue.[16,21,22] CD44v6, a glycosylated cell surface adhesion molecule that is involved in cell–cell and cell–matrix interactions, can influence tumor cell invasion and metastatic behavior.[23–25] Both of them are shown to be correlated to tumor progression, lymph node metastasis, and prognosis. In the mediastinal group of patients in this research, 18 patients (37%) were positive for Ber-Ep4. Of these, 25 lymph nodes (16%) from 13 patients (27%) were also positive for CD44v6. A total of 28 lymph nodes (18%) from 16 patients (33%) were positive for CD44v6. Expression of CD44v6 and Ber-Ep4 was positively correlated (r = 0.643, P < 0.001), though we cannot find any literature about their connection. More researches are needed to confirm the connection among them. We, therefore, conclude that both Ber-Ep4 and CD44v6 are valuable markers for detecting disseminated tumor cells in AEJ tumors by IHC.
Molecular examination [reverse transcription-polymerase chain reaction (RT-PCR)] can be also used to detect LNMM. Cytokeratin and carcinoembryonic antigen are often used as target markers.[26,27] It shows a satisfactory degree of sensitivity and specificity. Some studies demonstrated that RT-PCR assay outperformed IHC in the detection of LNMM.[27,28] Moreover, Kubota et al[29] also reported the higher sensitivity of RT-PCR assay than IHC. But Ruud et al[30] pointed out that false-positive results might be produced with the presence of impureness or pseudogene. Meanwhile, because of the heterogeneous expression of target markers, there could be a possibility of false negativity.[31]
The clinical relevance of LNMM is still a controversial issue. Schurr et al[18] reported that LNMM was significantly associated with depth of invasion and N category, but not with age, tumor size, or degree of differentiation. Xie et al[20] also reported that depth of invasion and lymph nodal status were related to LNMM. Our study showed that Lauren type was also a risk factor (P = 0.038 and 0.002). They identified LNMM as an independent prognostic factor for disease-specific survival and proposed that IHC should be added into conventional HE to clarify patients who are at risk. Bonavina et al[10] and Mueller et al[32] also concluded that patients without LNMM would have a significant survival benefit. The prognostic impact was also observed and confirmed in esophageal and gastric carcinoma.[16,33,34] We also found that patients showed a significant survival benefit for those with negative LNMM (P < 0.05). Positive CD44v6 group had a significantly reduced survival (P < 0.001). Patients with positive Ber-Ep4 cells had a lower disease-specific survival, though not statistically significant (P = 0.058). This may be a consequence of the limited number of patients. On the contrary, Horstman et al[35] said that LNMM was not correlated to any clinicopathological factors. Some studies showed that the presence of micrometastasis had no influence on survival time.[36,37] However, it is still indistinct whether the presence of individual or small clusters of micrometastasis tumor cells has a significant prognostic value.
Surgical resection is still the main treatment for those patients with AEJ. According to Siewert classification, subtypes differ obviously in terms of surgical therapy, histogenesis, and clinicopathological characteristics. For Siewert type I AEJ,[7] transthoracic approach is accepted and enables mediastinal lymph node dissection, which is not obtained through the esophageal hiatus. Thorough mediastinal dissection and esophagectomy are necessary. For Siewert type III AEJ,[8] transthoracic approach does not warrant a significant survival benefit, but observes a higher morbidity. Transhiatal total gastrectomy with dissection of the distal esophagus is counseled. It is difficult to obtain sufficient margins when tumor invades the distal esophagus more than 3 cm beyond the EGJ. In this situation, it is necessary to perform an aggressive lower mediastinal lymph node resection.[11]
The optimal surgical approach and extent of lymph node dissection is still unclear for type II AEJ.[7,8,38] Some authors prefer transthoracic approach with mediastinal lymphadenectomy, whereas the others choose transhiatal. It is controversial which one is the best conjunctive operation among esophagectomy, gastrectomy, and esophagogastrectomy. A Japanese group showed the evidence that thoracotomy did not provide significant benefit, but leads to higher morbidity in comparison to the transhiatal approach.[8] But Schurr et al[18] and Parry et al[39] reported the high prevalence of mediastinal nodal metastasis and a strong prognosticator of positive mediastinal lymph nodes. They suggested an aggressive lower mediastinal lymphadenectomy with R0 resection especially in cases of esophageal invasion with more 30 mm.[11] In mediastinal cohort, we found that 12 positive lymph nodes (8%) in 7 out of 49 patients (14%) were positive in HE staining. While, stained with LNMM, it was 12 positive lymph nodes (8%) in 21 out of 49 patients (43%). When combining LNMM with HE staining, the incidence of positive mediastinal lymph nodes was increased to 37 lymph nodes (24%) in 28 patients (57%). Patients with positive LNMM had a reduced disease-specific survival, with significance (P < 0.05). In 42 out of 49 patients (86%) with negative lower mediastinal lymph nodes tested by conventional examination, we still found that no LNMM group obtained significant benefit (P < 0.05). Therefore, there is a possibility of potential metastasis to lymph nodes of mediastinal compartment. The lymph node dissection of lower mediastinal seems to be necessary.
There were some limitations in our study; this analysis was based on a small sample size at a single center. The number of pN0 patients was small. A randomized, multicenter controlled cohort of AEJ patients is needed to confirm our results.
5. Conclusion
Our study demonstrated that it is necessary to test for LNMM within those negative lymph node patients in routine examination. As a positive factor, thorough lower mediastinal lymphadenectomy in an invasive approach should be considered when necessary. Ber-Ep4 and CD44v6 were shown to be great markers for detecting LNMM.
Acknowledgments
The authors thank pathologist Chang-Yin Fen and Guo-Dong Zhong for their generous help. We also thank Rong Fu for her help in reviewing statistical methods and results.
Footnotes
Abbreviations: AEJ = adenocarcinoma of the esophagogastric junction, EGJ = esophagogastric junction, IHC = immunohistochemistry, LNMM = lymph node micrometastasis.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Sehdev A, Catenacci DV. Gastroesophageal cancer: focus on epidemiology, classification, and staging. Discov Med 2013;16:103–11. [PubMed] [Google Scholar]
- [2].Hasegawa S, Yoshikawa T. Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric cancer 2010;13:63–73. [DOI] [PubMed] [Google Scholar]
- [3].Rudiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1002 consecutive patients. Ann Surg 2000;232:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feith M, Stein HJ, Siewert JR. Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am 2006;15:751–64. [DOI] [PubMed] [Google Scholar]
- [5].Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: relevance of a topographic-anatomic subclassification. J Surg Oncol 2005;90:139–46. discussion 46. [DOI] [PubMed] [Google Scholar]
- [6].Kurokawa Y, Sasako M, Doki Y. Treatment approaches to esophagogastric junction tumors. Dig Surg 2013;30:169–73. [DOI] [PubMed] [Google Scholar]
- [7].Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662–9. [DOI] [PubMed] [Google Scholar]
- [8].Sasako M, Sano T, Yamamoto S, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 2006;7:644–51. [DOI] [PubMed] [Google Scholar]
- [9].Hosokawa Y, Kinoshita T, Konishi M, et al. Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan. Ann Surg Oncol 2012;19:677–83. [DOI] [PubMed] [Google Scholar]
- [10].Bonavina L, Ferrero S, Midolo V, et al. Lymph node micrometastases in patients with adenocarcinoma of the esophagogastric junction. J Gastrointest Surg 1999;3:468–76. [DOI] [PubMed] [Google Scholar]
- [11].Hosoda K, Yamashita K, Katada N, et al. Impact of lower mediastinal lymphadenectomy for the treatment of esophagogastric junction carcinoma. Anticancer Res 2015;35:445–56. [PubMed] [Google Scholar]
- [12].Rutgers EJ. Sentinel node micrometastasis in breast cancer. Br J Surg 2004;91:1241–2. [DOI] [PubMed] [Google Scholar]
- [13].Arigami T, Uenosono Y, Yanagita S, et al. Clinical significance of lymph node micrometastasis in gastric cancer. Ann Surg Oncol 2013;20:515–21. [DOI] [PubMed] [Google Scholar]
- [14].Yasuda K, Adachi Y, Shiraishi N, et al. Pattern of lymph node micrometastasis and prognosis of patients with colorectal cancer. Ann Surg Oncol 2001;8:300–4. [DOI] [PubMed] [Google Scholar]
- [15].Wang J, Yu JC, Kang WM, et al. The predictive effect of cadherin-17 on lymph node micrometastasis in pN0 gastric cancer. Ann Surg Oncol 2012;19:1529–34. [DOI] [PubMed] [Google Scholar]
- [16].Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970–5. [DOI] [PubMed] [Google Scholar]
- [17].Sobin LH, Wittekind C. International Union against Cancer: TNM: Classification of Malignant Tumors. 7th edWest Sussex: Wiley-Blackwell; 2010. [Google Scholar]
- [18].Schurr PG, Yekebas EF, Kaifi JT, et al. Lymphatic spread and microinvolvement in adenocarcinoma of the esophago-gastric junction. J Surg Oncol 2006;94:307–15. [DOI] [PubMed] [Google Scholar]
- [19].Ru Y, Zhang L, Chen Q, et al. Detection and clinical significance of lymph node micrometastasis in gastric cardia adenocarcinoma. J Int Med Res 2012;40:293–9. [DOI] [PubMed] [Google Scholar]
- [20].Xie JW, Chen PC, Zheng CH, et al. Evaluation of the prognostic value and functional roles of CD44v6 in gastric cancer. J Cancer Res Clin Oncol 2015;141:1809–17. [DOI] [PubMed] [Google Scholar]
- [21].Latza U, Niedobitek G, Schwarting R, et al. Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol 1990;43:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Momburg F, Moldenhauer G, Hammerling GJ, et al. Immunohistochemical study of the expression of a Mr 34,000 human epithelium-specific surface glycoprotein in normal and malignant tissues. Cancer Res 1987;47:2883–91. [PubMed] [Google Scholar]
- [23].Okayama H, Kumamoto K, Saitou K, et al. CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for prediction of lymph node metastasis in primary gastric cancer. Oncol Rep 2009;22:745–55. [DOI] [PubMed] [Google Scholar]
- [24].Pitule P, Cedikova M, Daum O, et al. Immunohistochemical detection of cancer stem cell related markers CD44 and CD133 in metastatic colorectal cancer patients. BioMed Res Int 2014;2014:432139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang J, Chang B, Liu J. CD44 standard form expression is correlated with high-grade and advanced-stage ovarian carcinoma but not prognosis. Hum Pathol 2013;44:1882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Okada Y, Fujiwara Y, Yamamoto H, et al. Genetic detection of lymph node micrometastases in patients with gastric carcinoma by multiple-marker reverse transcriptase-polymerase chain reaction assay. Cancer 2001;92:2056–64. [DOI] [PubMed] [Google Scholar]
- [27].Matsumoto M, Natsugoe S, Ishigami S, et al. Lymph node micrometastasis and lymphatic mapping determined by reverse transcriptase-polymerase chain reaction in pN0 gastric carcinoma. Surgery 2002;131:630–5. [DOI] [PubMed] [Google Scholar]
- [28].Arigami T, Natsugoe S, Uenosono Y, et al. Lymphatic invasion using D2-40 monoclonal antibody and its relationship to lymph node micrometastasis in pN0 gastric cancer. Br J Cancer 2005;93:688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kubota K, Nakanishi H, Hiki N, et al. Quantitative detection of micrometastases in the lymph nodes of gastric cancer patients with real-time RT-PCR: a comparative study with immunohistochemistry. Int J Cancer 2003;105:136–43. [DOI] [PubMed] [Google Scholar]
- [30].Ruud P, Fodstad O, Hovig E. Identification of a novel cytokeratin 19 pseudogene that may interfere with reverse transcriptase-polymerase chain reaction assays used to detect micrometastatic tumor cells. Int J Cancer 1999;80:119–25. [DOI] [PubMed] [Google Scholar]
- [31].Kuo CT, Hoon DS, Takeuchi H, et al. Prediction of disease outcome in melanoma patients by molecular analysis of paraffin-embedded sentinel lymph nodes. J Clin Oncol 2003;21:3566–72. [DOI] [PubMed] [Google Scholar]
- [32].Mueller JD, Stein HJ, Oyang T, et al. Frequency and clinical impact of lymph node micrometastasis and tumor cell micro involvement in patients with adenocarcinoma of the esophagogastric junction. Cancer 2000;89:1874–82. [DOI] [PubMed] [Google Scholar]
- [33].Yasuda K, Adachi Y, Shiraishi N, et al. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol 2002;9:771–4. [DOI] [PubMed] [Google Scholar]
- [34].Sonoda H, Tani T. Clinical significance of molecular diagnosis for gastric cancer lymph node micrometastasis. World J Gastroenterol 2014;20:13728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Horstmann O, Fuzesi L, Markus PM, et al. Significance of isolated tumor cells in lymph nodes among gastric cancer patients. J Cancer Res Clin Oncol 2004;130:733–40. [DOI] [PubMed] [Google Scholar]
- [36].Choi HJ, Kim YK, Kim YH, et al. Occurrence and prognostic implications of micrometastases in lymph nodes from patients with submucosal gastric carcinoma. Ann Surg Oncol 2002;9:13–9. [DOI] [PubMed] [Google Scholar]
- [37].Glickman JN, Torres C, Wang HH, et al. The prognostic significance of lymph node micrometastasis in patients with esophageal carcinoma. Cancer 1999;85:769–78. [PubMed] [Google Scholar]
- [38].von Rahden BH, Stein HJ, Siewert JR. Surgical management of esophagogastric junction tumors. World J Gastroenterol 2006;12:6608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Parry K, Haverkamp L, Bruijnen RC, et al. Surgical treatment of adenocarcinomas of the gastro-esophageal junction. Ann Surg Oncol 2015;22:597–603. [DOI] [PubMed] [Google Scholar]


