Figure 2.
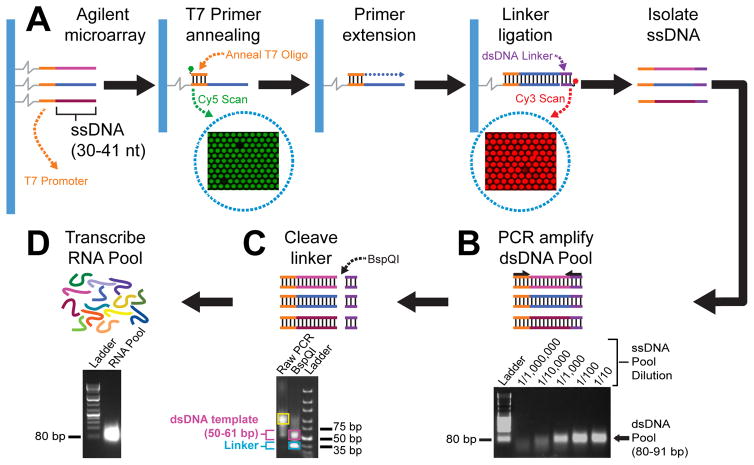
Custom DNA and RNA pool generation from microarray. A) Agilent microarrays contain ssDNA probes that have variable designed sequences (multicoloured) and a T7 promoter sequence (orange lines). A Cy3 (green circle) labeled T7 promoter primer (orange line) is annealed to ssDNA microarray probes which are subsequently double-stranded by enzymatic primer extension using T4 DNA polymerase (blue dotted line). A dsDNA linker (purple lines), labeled with Cy5 (red circle), is ligated to the dsDNA microarray probes using T4 DNA ligase. Cy3 and Cy5 signals are detected on microarrays by scanning at 532 and 635 nm, respectively, to verify efficient T7 primer annealing and linker ligation. Scanned Cy3 and Cy5 microarray images are shown (enclosed by dotted blue circles). B) PCR amplification of the dsDNA pool using ssDNA pool purified from the microarray as template. ssDNA pool (PCR template) dilutions as well as bands corresponding to an 80 bp DNA ladder and a 80–91 bp dsDNA pool are indicated on the ethidium bromide stained agarose gel image. C) Linker cleavage is mediated by a Type IIS restriction enzymes, BspQI. Representative bands corresponding to undigested PCR amplified dsDNA pool (enclosed by yellow box), BspQI digested dsDNA template (enclosed by pink box), and cleaved dsDNA linker (enclosed by blue box) are indicated. D) The non-random RNA pool (multicoloured lines and high intensity band in the agarose gel image) is generated via T7 RNA polymerase mediated transcription reactions, using gel purified dsDNA templates (shown in panel 2C).