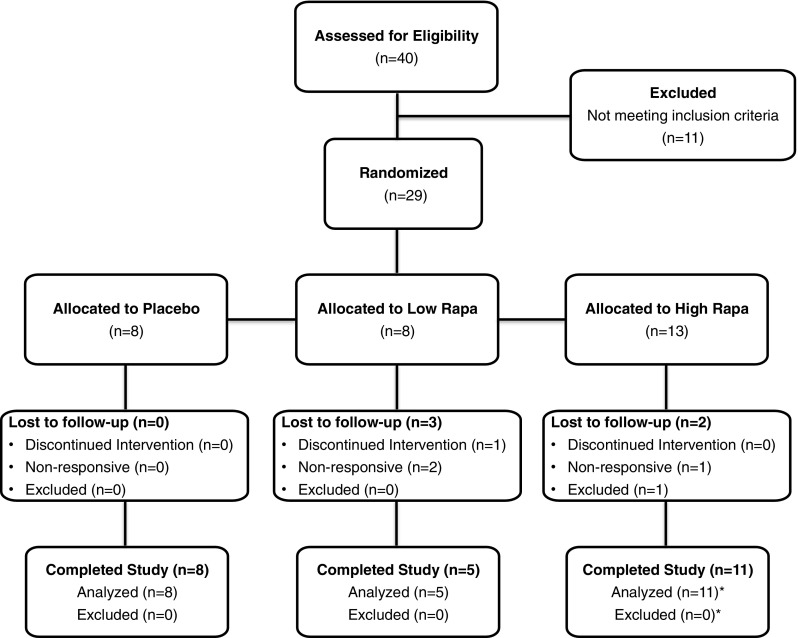
Fig. 1.
Dog Aging Project Rapamycin Intervention Phase I Trial Flow Diagram. Forty dogs were assessed for eligibility at the first veterinary visit, and 11 dogs were excluded due to pre-existing conditions that were inconsistent with the study protocol. Twenty-nine dogs were allocated into treatment groups according to the pre-determined randomization schedule based on their chronological order of entry into the study. Two dogs were lost to follow-up in the low rapamycin group because the owners failed to complete the required veterinary visits. One dog was lost to follow-up in the low rapamycin group because the owner discontinued the medication. Two dogs were lost to follow-up in the high rapamycin group because one owner failed to complete the required veterinary visits and it was discovered during the study period that one owner failed to disclose a pre-existing skin condition requiring medication, resulting in removal of this dog from the study. Asterisk signs indicate a dog in the high-dose rapamycin group completed the entire 10-week study, but it was discovered upon reviewing the dosing log that the owners had mistakenly provided the dog with 0.025 mg/kg three times each week, one quarter the intended dose of the study drug. This dog was removed from analysis of the high rapamycin group, but was included in analysis of the pooled (low and high dose) rapamycin-treated dogs