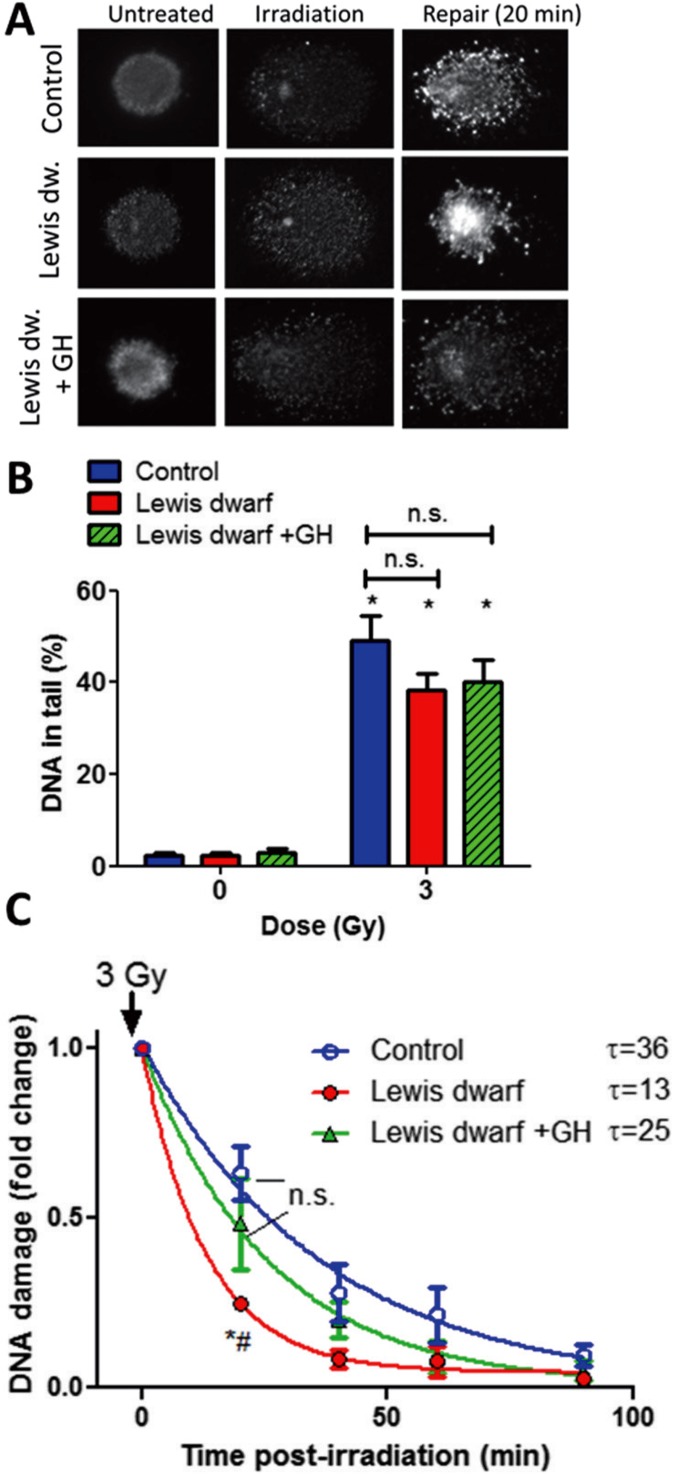
Fig. 1.
Early-life GH/IGF-1 status of donor rats elicits persisting alterations in DNA repair capacity in cultured primary fibroblasts. Fibroblasts were γ-irradiated, and DNA damage was assessed by single-cell electrophoresis (“comet assay”) at different time points post-irradiation. Damaged DNA migrates during electrophoresis from the nucleus toward the anode, forming the shape of a “comet” with a head (cell nucleus with intact DNA) and a tail (relaxed and broken DNA). a Representative images of damaged DNA in irradiated (3 Gy) fibroblasts derived from control rats, Lewis dwarf rats, and Lewis dwarf rats with early-life GH treatment. Median values of tail DNA content in each sample were determined. Note the marked decline in tail DNA content at 20 min post-irradiation due to DNA repair processes. b γ-Irradiated fibroblasts exhibit comparable DNA damage. Shown are γ-irradiation (3 Gy)-induced increases in tail DNA content in fibroblasts derived from control rats, Lewis dwarf rats, and Lewis dwarf rats with peripubertal GH treatment. *p < 0.05 vs respective non-irradiated controls (one-way ANOVA, Tukey’s multiple comparisons). c Fibroblasts derived from control rats Lewis dwarf rats exhibit increased DNA repair capacity, which is prevented by peripubertal GH treatment. For assessment of DNA repair efficiency, the percentage of residual DNA damage was plotted as a function of time post-irradiation. Tail DNA content at each time point is shown. The residual DNA damage at 20 min post-irradiation was the lowest in fibroblasts from untreated Lewis dwarf rats, indicating more efficient DNA repair processes in these cells, which was reversed by peripubertal GH treatment. *p < 0.001 Lewis dwarf vs control; # Lewis dwarf vs Lewis dwarf GH (one-way ANOVA, Tukey’s multiple comparisons); data are mean ± SEM (n = 5–6 for each time-point). The time constant calculated from these plots (τ) is shown next to the legends