FIG 6.
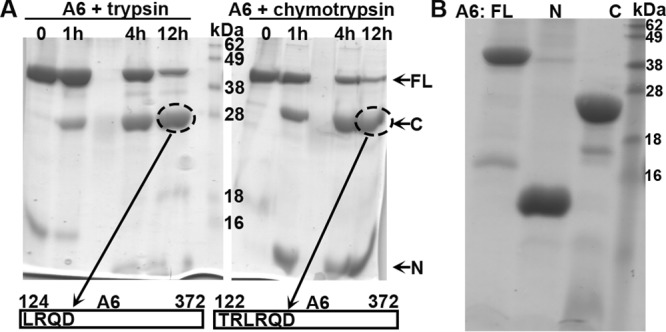
(A) Analysis of A6 domain architecture by limited proteolysis. The purified recombinant A6 protein at 2 mg/ml was mixed with trypsin (left) or chymotrypsin (right) at a 1,000:1 mass ratio and incubated at room temperature. Samples at various time points (indicated) were analyzed by SDS-PAGE. The Coomassie blue-stained gel image is shown in black. Note the stable fragment of about 28 kDa (circled) after overnight proteolysis in both cases. The fragments were excised from the gel and subjected to tandem mass spectrometry and N-terminal peptide sequencing analysis. The N-terminal sequences of the fragments were determined to be LRQD and TRLR, which are the starting sequences for A6 aa 124 to 372 and aa 122 to 372, respectively. (B). Purification of recombinant full-length (FL) A6, the N domain of A6 (N) (aa 1 to 121), and the C domain of A6 (C) (aa 122 to 372). The recombinant A6 proteins were expressed as a SUMO fusion with an N-terminal 6×His tag in E. coli and purified as described in Materials and Methods. The purified A6 proteins with the tag removed were analyzed by SDS-PAGE and stained with Coomassie blue (shown in black).
