ABSTRACT
Viruses infecting the Archaea harbor a tremendous amount of genetic diversity. This is especially true for the spindle-shaped viruses of the family Fuselloviridae, where >90% of the viral genes do not have detectable homologs in public databases. This significantly limits our ability to elucidate the role of viral proteins in the infection cycle. To address this, we have developed genetic techniques to study the well-characterized fusellovirus Sulfolobus spindle-shaped virus 1 (SSV1), which infects Sulfolobus solfataricus in volcanic hot springs at 80°C and pH 3. Here, we present a new comparative genome analysis and a thorough genetic analysis of SSV1 using both specific and random mutagenesis and thereby generate mutations in all open reading frames. We demonstrate that almost half of the SSV1 genes are not essential for infectivity, and the requirement for a particular gene correlates well with its degree of conservation within the Fuselloviridae. The major capsid gene vp1 is essential for SSV1 infectivity. However, the universally conserved minor capsid gene vp3 could be deleted without a loss in infectivity and results in virions with abnormal morphology.
IMPORTANCE Most of the putative genes in the spindle-shaped archaeal hyperthermophile fuselloviruses have no sequences that are clearly similar to characterized genes. In order to determine which of these SSV genes are important for function, we disrupted all of the putative genes in the prototypical fusellovirus, SSV1. Surprisingly, about half of the genes could be disrupted without destroying virus function. Even deletions of one of the known structural protein genes that is present in all known fuselloviruses, vp3, allows the production of infectious viruses. However, viruses lacking vp3 have abnormal shapes, indicating that the vp3 gene is important for virus structure. Identification of essential genes will allow focused research on minimal SSV genomes and further understanding of the structure of these unique, ubiquitous, and extremely stable archaeal viruses.
KEYWORDS: Archaea, morphogenesis, mutational studies, virus assembly
INTRODUCTION
The Archaea, particularly hyperthermophilic Archaea that grow optimally above 80°C, are infected by some of the most structurally and genetically diverse viruses known (reviewed by Prangishvili [1]). Of the numerous morphologically unique viruses, those possessing spindle-shaped architectures are widespread within the Archaea and include some of the best-characterized archaeal viruses. Spindle-shaped archaeal viruses belong to two viral families, the Fuselloviridae and Bicaudaviridae, which have few similarities other than overall capsid morphology (2). Fuselloviruses have been isolated worldwide from volcanic hot spring environments (70 to 80°C, pH ∼2 to 4) in which their hosts, Sulfolobus solfataricus and its close relatives, thrive (1, 3–6).
Sulfolobus spindle-shaped virus 1 (SSV1) and its host, S. shibatae, originally were isolated from a hot spring in Beppu, Japan (3). SSV1 encapsidates a circular, positively supercoiled 15.5-kbp double-stranded DNA (dsDNA) genome (7, 8). Transcription of the SSV1 genome following UV irradiation, which also leads to virion production, proceeds via early and late transcripts (9, 10) (Fig. 1). The transcripts in the SSV1 genome carry 35 open reading frames (ORFs), most of which cannot be assigned functions due to undetectable homology with sequences in public databases (11). This situation is not unique among viruses; however, it seems especially pronounced among crenarchaeal viruses and hinders our understanding of these viruses and their life cycles (12–14). All fuselloviruses encode a tyrosine family recombinase or integrase that facilitates virus genome integration into a host tRNA gene. However, the viral integrase gene does not appear to be required for virus infectivity (15). To date, only the SSV1 integrase and structural proteins (VP1, VP2, VP3, and VP4) have been assigned functions, although a combination of structural studies and bioinformatics have provided predictions for the roles of several others (16).
FIG 1.
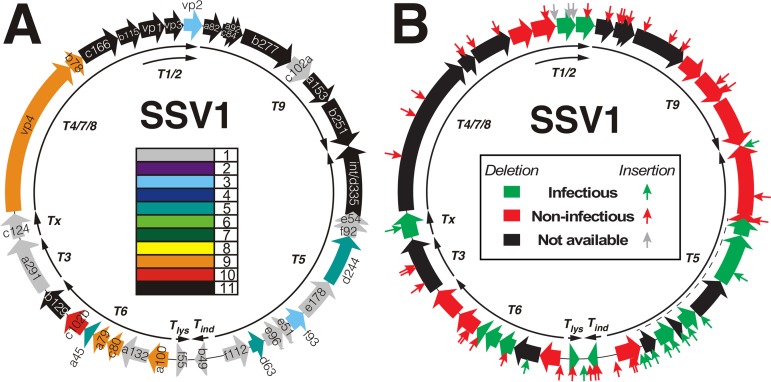
Annotated SSV1 genome and mutation summary. (A) Conserved ORFs in SSV1. Open reading frames (ORFs) are displayed as block arrows and labeled as described in Palm et al. (7). The conservation of each SSV1 ORF relative to 10 other fusellovirus genomes is indicated by the color code in the middle of the map. Color coding is from black, present in all 11 genomes, to gray, present only in the SSV1 genome. Conserved ORFs were identified by BLAST-P (34) using an E value of <0.001 to identify putative homologs. Core SSV1 ORFs, black, are labeled in white text, and all others are in black text. Mapped viral transcripts (9, 10, 25) are shown as thin black arrows on the interior of the map and are labeled. (B) SSV1 mutants. The ORF fill color indicates if deletion of the ORF in the genome allows production of infectious virus (green) or not (red) when transformed into S. solfataricus strain S441. ORFs for which deletions are not available are filled in black. The dotted line near the T5 transcript indicates the region deleted in the infectious EAI582 mutant. Each of the small arrows perpendicular to the map denotes the location of an EZ-Tn5 transposon insertion. The color of the arrow indicates whether the virus genome containing that insertion results in the production of infectious virus (green) or not (red) when transformed into S. solfataricus strain S441. Gray arrows in the vp1/vp3 gene region indicate insertions that apparently are removed via recombination, allowing the production of infectious virus (see the text). Transcripts are as described for panel A. All noninfectious mutants labeled in red have been tested independently at least 5 times.
Fusellovirus capsids are comprised of the major capsid protein VP1, host-derived lipids, and smaller amounts of the minor capsid proteins VP3 and VP4 (17, 18). The VP1 and VP3 proteins are highly conserved within the Fuselloviridae and are clearly homologous to each other (11, 17). SSV1 and three other fuselloviruses, SSV6, Acidianus spindle-shaped virus 1 (ASV1), and Sulfolobus Mexican fusellovirus 1 (SMF1), also encode a small and extremely basic structural protein (VP2) that is thought to bind to viral DNA within the capsid (19). VP2 has been found in SSV1 virions (17, 18). A fourth capsid protein, VP4 (formerly SSV1-C792), has been identified via mass spectrometry of purified virions and likely forms the tail fiber of the virion (18, 20). Recently it was shown that purified SSV1 virions contain host-derived glycerol dibiphytanyl glycerol tetraether (GDGT) lipids (18). The structure of the SSV1 virion has been solved via cryoelectron microscopy, providing insight into the architecture and assembly of this diverse family of viruses (21).
A number of putative transcription factors have been identified in the SSV1 genome, none of which have been experimentally characterized. Atomic resolution structures of SSV1-B129, SSV1-F112, SSV1-F93, and SSV8-E73 (a homologue of SSV1-E51) reveal DNA binding domains characteristic of transcriptional regulators (16, 22–24). The products of four other well-conserved SSV1 ORFs (SSV1-A45, SSV1-A79, SSV1-C80, and SSV1-B115) are predicted to encode ribbon-helix-helix (RHH), helix-turn-helix (HTH), or zinc finger (ZNF) DNA binding domains and may also be involved in transcription regulation (12). The protein product of ORF f55 is predicted to possess an RHH DNA binding domain and was shown to bind weakly to a number of viral promoters in vitro that presumably control the expression of early gene products. Based on these data, SSV1-F55 is hypothesized to maintain lysogeny by repressing early viral promoters (25, 26).
SSV1-B251 and SSV1-A153 are highly conserved and are the only fusellovirus proteins encoded by the satellite nucleic acid pSSVx, whose genome can be packaged in smaller virus capsids upon infection by a bona fide helper SSV. This result led to the prediction that the SSV1-B251 and SSV1-A153 ORFs are involved in replication or packaging (27). SSV1-B251 possesses NTP binding motifs and is predicted to be homologous to the bacterial dnaA gene (28). The structure of SSV8-D212, a homologue of SSV1-D244, has a predicted nuclease fold, although activity was not demonstrated biochemically (20). SSV1-D244 was identified in one study by mass spectrometry of purified SSV1 virions, but it was not detected in a later analysis (18, 20). The structure of SSV1-D63 displays a four-helix bundle that is characteristic of a large number of proteins, making a functional prediction difficult (29).
SSV1 is one of only two archaeal viruses amenable to genetic study (30, 31). The ability of SSV1 to tolerate large insertions of foreign DNA allowed the construction of SSV1-based shuttle vectors and provided tools for studying the viral genome itself (30, 32). Partial digestion of SSV1 DNA followed by the insertion of an Escherichia coli plasmid provided the first data on which genes are required for virus infectivity (30). These data indicated that a majority of ORFs in SSV1 are essential for virus function.
SSV1 shuttle vectors were essential for subsequent work in which specific ORFs were deleted from the viral genome. Using long inverse PCR (LIPCR), the SSV1 integrase gene was deleted and subsequently shown not to be essential for production of infectious virus in S. solfataricus P2 (15). More recently, in a structure-guided study, the vp2 gene and ORFs b129 and d244 were deleted (11). Deletion of b129, a putative transcriptional regulator, resulted in loss of infectivity, whereas removal of the putative DNA binding protein gene vp2 and predicted nuclease gene d244 resulted in production of infectious virus. Homologues of SSV1-B129 have been identified in the genomes of all 11 isolated fuselloviruses, indicating that it is important for virus infectivity (Fig. 1A). SSV1-VP2 is much less well conserved, which may explain why it can be deleted without a loss of infectivity (Fig. 1). However, host-encoded chromatin proteins that have been found in purified virions may be able to functionally complement this lack of the vp2 gene (18). Interestingly, cells that were infected with SSV1-Δd244 exhibited a retarded growth phenotype compared to cells infected with wild-type SSV1 or SSV1-Δvp2 (11).
To further characterize the genetic requirements for SSV1 function, LIPCR and transposon mutagenesis were employed to construct 78 mutant SSV1 genomes harboring mutations in each of the 35 ORFs. SSV1 appears to be much more tolerant of mutagenesis than previously thought, as half of the genes could be mutated without abrogating infectivity. Almost the entirety of the T5 early transcript appears to be expendable, while the T6 early transcript is much less so. This correlates with the abundance of well-conserved fusellovirus genes in the T6 transcript. The genes of the newly defined fusellovirus core appear to be essential with the surprising exception of the minor capsid gene vp3, which was shown not to be essential for SSV1 infectivity.
(Parts of this research, namely, the mutagenesis results and modified versions of Fig. 1 to 4A, were previously published as part of Eric A. Iverson's Ph.D. dissertation [33].)
FIG 4.
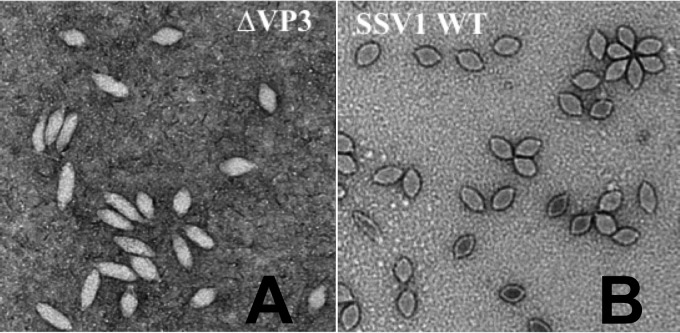
SSV1 virion electron micrographs. (A) Typical transmission electron micrographs of SSV1-Δvp3 (EAI420) virions. (B) Typical transmission electron micrographs of SSV1 wild-type virions. SSV1 wild-type particles are ∼76 by 40 nm. Samples were negatively stained with uranyl acetate and imaged on a Tecnai F-20 TEM (FEI Inc.) at a 200-keV accelerating voltage. Images were obtained with a Gatan Ultrascan camera.
RESULTS
Identification of fusellovirus core genes.
In order to understand gene conservation in SSV1 and provide context for our mutagenesis results, all SSV1 ORFs were compared to all other full-length circular SSV genomes from virion-containing cultures using BLAST-P (34). The SSVs used were SSV2, SSV3, SSV4, SSV5, SSV6, SSV7, SSV8, SSV9, ASV, and SSVL (citations are listed in Table 1). Putative homologs were identified as having a BLAST-P E score of < 10−3 using the SSV1 ORF as a query. This analysis identified a set of 12 genes/ORFs, the fusellovirus core, that are conserved in all Fuselloviridae (black arrows with white lettering in Fig. 1A) (Table 2).
TABLE 1.
Fusellovirus genomes used in this study
| Virus | Genome size (bp) | No. of ORFs | GenBank accession no. | Reference or source |
|---|---|---|---|---|
| SSV1 | 15,465 | 35 | NC_001338 | 7 |
| SSV2 | 14,795 | 35 | NC_005265 | 4 |
| SSV3 | 15,230 | 32 | KY579375 | 35 |
| SSV4 | 15,135 | 34 | NC_009986 | 36 |
| SSV5 | 15,330 | 34 | NC_011217 | 5 |
| SSV6 | 15,684 | 33 | NC_013587 | 5 |
| SSV7 | 17,602 | 33 | NC_013588 | 5 |
| SSV8a | 16,473 | 37 | NC_005360 | 6 |
| SSV9b | 17,385 | 31 | NC_005361 | 6 |
| SSVL | 16,271 | 36 | KY563228 | Personal communication |
| ASV1 | 24,186 | 38 | NC_013585 | 5 |
SSV8 formerly referred to as Sulfolobus spindle-shaped virus Ragged Hills.
SSV9 formerly referred to as Sulfolobus spindle-shaped virus Kamchatka.
TABLE 2.
Fusellovirus core genes
| SSV1 gene | Annotation | Reference |
|---|---|---|
| vp1 | Major capsid protein | 17 |
| vp3 | Minor capsid protein | 17 |
| integrase | Viral integrase | 37 |
| a153 | None (conserved in SSVx) | |
| a82 | None | |
| a92 | None | |
| b115 | Putative HTH transcription regulator | 12 |
| b129 | Putative C2H2 ZNF transcription regulator | 16 |
| b251 | ATPase, DnaA homologue, lon-like protease (conserved in pSSVx) | 28 |
| b277 | None | |
| c84 | None | |
| c166 | None |
Summary of SSV1 mutagenesis.
To better understand the function of SSV1 genetic elements, both specific and random mutagenesis of SSV1 were performed. All 35 ORFs in the SSV1 genome were mutated via insertion and/or deletion (Table 3), and all 78 of these mutants were tested for their ability to infect S. solfataricus S441, a host permissive and susceptible to infection by all SSVs tested to date (38). Insertion mutants in intergenic regions were also isolated and characterized. Infectivity of SSV1 mutants was assayed by transformation of purified mutant DNA into uninfected Sulfolobus cells and then spotting these cultures onto an uninfected lawn. Spotting a productively infected culture on a host lawn results in the appearance of a ring of growth inhibition, or halo, around the spot after 48 to 72 h of incubation at 75°C (11, 38). Transformation of many SSV1 mutants into uninfected Sulfolobus did not result in cultures that inhibited growth of uninfected Sulfolobus on lawns, indicating that the mutation generated a defective virus genome (red arrows and ORFs in Fig. 1B). To lower the frequency of false negatives, a mutant was only characterized as noninfectious after a minimum of 5 independent negative halo assay results. The most frequently used positive control, mutant REC262, produced a halo in 97% of transformations.
TABLE 3.
SSV1 mutants
| Plasmid | Description | Infectious in s441 (+ or −) | Reference or source |
|---|---|---|---|
| pAJC96 | pAJC97 background with integrase (ORF d335) deleted (also called SSV1-Δint) | − | 15 |
| pAJC97 | SSV1 shuttle vector (TOPO PCR blunt II inserted at bp 3173) (ORF e178) | + | 15 |
| REC228a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 4680 (ORF f112) | + | This work |
| REC229a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 11265 (vp4) | − | This work |
| REC230a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 8277 (ORF a291) | − | This work |
| REC231a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 11227 (vp4) | − | This work |
| EAI232 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 8662 (ORF a291) | − | This work |
| EAI239 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5218 (ORF b49) | − | This work |
| EAI240 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 4792 (ORF f112) | − | This work |
| EAI241 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 100 (ORF a100) | − | This work |
| EAI242 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 10159 (vp4) | − | This work |
| REC243a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 14046 (ORF c84/a82) | − | This work |
| REC244a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 4788 (ORF f112) | − | This work |
| REC245a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 12718 (vp1) | − | This work |
| EAI247 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 13992 (ORF c84/a82) | − | This work |
| EAI248 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 953 | − | This work |
| EAI249 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 6375 (ORF a132) | + | This work |
| EAI250 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 11641 (ORF b78) | − | This work |
| EAI251 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 14677 (ORF b277) | − | This work |
| EAI253 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 3889 (ORF e51) | + | This work |
| EAI254 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 8633 (ORF a291) | − | This work |
| EAI255 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 7509 (ORF b129) | − | This work |
| EAI256 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 2776 (ORF d244) | + | This work |
| EAI257 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 4249 (ORF e96) | + | This work |
| EAI258 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 8998 (ORF c124) | + | This work |
| EAI260 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 80 (ORF a153) | − | This work |
| EAI261 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 14209 (ORF b277) | − | This work |
| REC262a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 4209 (ORF e96) | + | This work |
| EAI266 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 1988 (ORF e54) | − | This work |
| EAI267 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 1717 (integrase) | − | This work |
| EAI271 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 6018 (ORF a100) | − | This work |
| EAI278 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 14834 (ORF b277) | − | This work |
| EAI281 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 13709 (ORF c84/a82) | − | This work |
| EAI282 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 11807 (ORF c166) | − | This work |
| EAI283 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 3572 (ORF e178) | + | This work |
| EAI286 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 11407 (vp4) | − | This work |
| EAI296 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5783 | − | This work |
| EAI297 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 12170 (ORF c166) | − | This work |
| EAI305 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 7359 (ORF c102b) | − | This work |
| EAI319 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 7387 (ORF c102b) | − | This work |
| REC322a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5573 (ORF f55) | − | This work |
| REC324a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 4394 (ORF d63) | + | This work |
| REC325a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 967 (integrase) | + | This work |
| EAI446 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 13211 (vp3) | +b | This work |
| EAI452 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 13003 | +b | This work |
| EAI453 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5451 | + | This work |
| EAI469 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 13491 (vp2) | − | This work |
| EAI476 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 13191 (vp3) | +b | This work |
| EAI477 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5247 (b49) | − | This work |
| EAI486 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5024 | + | This work |
| EAI492 | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 3837 (f92) | + | This work |
| EAI202 | pAJC97 background with ORF b129 deleted | − | This work |
| EAI201 | pAJC97 background with ORF d244 deleted | − | This work |
| EAI205 | pAJC97 background with ORF b49 deleted | + | This work |
| EAI206 | pAJC97 background with ORF b251 deleted | − | This work |
| EAI214 | pAJC97 background with ORF b115 deleted | − | This work |
| EAI216 | pAJC97 background with ORF e96 deleted | + | This work |
| EAI233 | pAJC97 background with ORF a100 deleted | − | This work |
| EAI327 | pAJC97 background with ORF f112 deleted | − | This work |
| EAI390 | pAJC97 background with ORF c124 deleted | + | This work |
| EAI394 | pAJC97 background with ORF a79 deleted | + | This work |
| EAI398 | pAJC97 background with ORF a45 deleted | + | This work |
| EAI400 | pAJC97 background with ORF f55 deleted | + | This work |
| EAI407 | pAJC97 background with ORF f92 deleted | + | This work |
| EAI413 | pAJC97 background with ORF f93 deleted | + | This work |
| EAI420 | pAJC97 background with vp3 deleted | + | This work |
| EAI421 | pAJC97 background with ORF c102a deleted | − | This work |
| EAI422 | pAJC97 background with C terminus of ORF b129 deleted | − | This work |
| EAI430 | pAJC97 background with N terminus of b129 deleted | − | This work |
| EAI435 | pAJC97 background with ORF e54 deleted | − | This work |
| EAI439 | pAJC97 background with ORF c80 deleted | + | This work |
| EAI496 | pAJC97 background with ORF c102b deleted | − | This work |
| EAI499 | pAJC97 background with ORF b129 deleted | − | This work |
| JAH572a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5016 | − | This work |
| JAH573a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5264 (b49) | − | This work |
| JAH576a | SSV1::Tn5 mutant, EZ-Tn5 inserted at bp 5681 | − | This work |
| EAI580 | EAI283 background with ORF d335 deleted | − | This work |
| EAI582 | EAI228 background with ORFs d63 through f92 deleted | + | This work |
Plasmids with the REC prefix refer to SSV1 mutants that were isolated in the Recombinant DNA Techniques Laboratory course at Portland State University. Plasmids with the JAH prefix refer to SSV1 mutants isolated by Jordan Hartunians.
Tn5 insertions in these mutants apparently were removed from Sulfolobus via homologous recombination.
Mutants in the mostly conserved ORFs in the T6 transcript.
The ∼2-kbp T6 transcript (9) encodes seven SSV1 ORFs (a100, a132, c80, a79, a45, c102b, and b129), which, with the exception of ORF a132, are well conserved within the Fuselloviridae (Fig. 1A). ORFs c80, a79, and a45 could be deleted, and ORF a132 tolerated transposon insertion without a loss of infectivity in any case. In contrast, the highly conserved ORFs c102b and a100 appeared to be essential for infectivity. Infectious virus was not produced when the transposon was inserted into ORFs a45 and c80, probably due to polar effects on essential ORFs c102b and b129. ORF b129 was previously determined to be essential for SSV1 infectivity (11). The structure of the SSV1-B129 protein has both N-terminal and C-terminal DNA binding domains, each of which has been shown to bind DNA (16 and personal communication). Mutants lacking ORF b129 amino acids (aa) 2 to 74 or 75 to 129 appear not to be capable of producing infectious virus, implying that the full-length SSV1-B129 protein is required for infectivity.
Mutants in the monocistronic transcripts T3 and Tx.
The monocistronic transcripts T3 and Tx are both expressed early after UV irradiation (10). The T3 transcript encodes SSV1 ORF a291, and Tx encodes SSV1 ORF c124 (Fig. 1A). None of three separate insertion mutants in ORF a291 resulted in the production of infectious virus (Fig. 1B). Conversely, ORF c124 tolerated both insertion in and deletion of the entire ORF without loss of infectivity. Interestingly, the ORF c124 insertion mutant produced infectious virus in only 3 of 12 independent transformations, which was not observed for other insertion mutants in ORFs or for the ORF c124 deletion mutant.
Fusellovirus core ORFs are intolerant of mutagenesis.
The completely conserved fusellovirus core gene ORFs c166, b115, a82, c84, a92, b277, a153, and b251 did not tolerate deletions and/or insertions (Fig. 1). The vp4 gene and ORF b78, both proposed to encode the SSV1 tail filament (5, 18), likewise appeared to be essential for infectivity. The only two ORFs in this region that are not well conserved in fuselloviruses are c102a and the structural gene vp2 (Fig. 1A). ORF c102a apparently is essential for infectivity. In contrast, the vp2 gene was previously shown to be nonessential (11). Unexpectedly, SSV1 lost infectivity when a transposon was inserted into the vp2 gene (Fig. 1B).
The T5 transcript region and ORFs therein are almost entirely dispensable.
The T5 transcript encodes a number of mostly nonconserved ORFs (Fig. 1A). This part of the SSV1 genome is extremely tolerant of mutation (Fig. 1B). Ten ORFs occupy this region, seven of which were shown to be nonessential (Fig. 1B). ORF f112 appeared to be essential, as it could not be deleted without abrogating infectivity. However, ORF f112 tolerated transposon insertion at amino acid 111 of the ORF but did not tolerate either of two insertions in the middle of the protein-coding sequence (Fig. 1B).
As the large stretch of nonessential ORFs occupying the T5 transcript indicated that the majority of this region was not required for SSV1 infectivity, we deleted a 2.4-kb region encompassing ORFs f92, d244, e178, f93, e51, e96, and d63 (Fig. 1B, dotted line). Somewhat surprisingly, SSV1 harboring this deletion remained infectious.
Integrase and e54 deletion mutants have a variable host range.
The integrase gene and ORF e54 are adjacent at the distal end of the T5 transcript (Fig. 1A). An SSV1 mutant lacking the integrase gene, pAJC96, also called SSV1-Δint (Table 3), was previously shown to be capable of infecting S. solfataricus strain P2 (15). We were unable to successfully infect Sulfolobus sp. strain S441 with SSV1-Δint but were able to productively infect Sulfolobus sp. strain Gϴ (Table 4) with SSV1-Δint. Similarly, SSV1 lacking ORF e54 was unable to infect Sulfolobus sp. strain S441 but was able to infect Sulfolobus sp. strain Gϴ. To confirm this result, a new integrase deletion was constructed in the genetic background of a Tn5 insertion in ORF e178, EAI283, also called SSV1-Δint2. This SSV1-Δint2 mutant has the same host range as the original SSV1-Δint virus. A mutant with a transposon inserted in ORF e54 did not productively infect Sulfolobus sp. strain S441 or Gϴ. An insertion in the integrase gene likewise inhibited SSV1 infectivity, although a mutant with a Tn5 insertion near the C terminus of the integrase was capable of producing virus in Sulfolobus sp. strain S441 (Fig. 1B).
TABLE 4.
Sulfolobus and E. coli strains used
| Strain | Description/genotype | Reference or source |
|---|---|---|
| S. solfataricus P1 | S. solfataricus isolate with complete genome sequence | DSM 1616 (39, 40) |
| S. solfataricus P2 | S. solfataricus isolate with complete genome sequence | DSM 1617 (41, 39) |
| S. solfataricus S441 | S. solfataricus isolate, SSV1 host | 38 |
| S. solfataricus GΘ | S. solfataricus MT4 derivative | 42 |
| E. coli EC100D pir+ | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ− rpsL (StrR) nupG pir+(DHFR) | Epicentre, Inc. |
| E. coli EC100D pir-116 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ− rpsL (StrR) nupG pir-116(DHFR) | Epicentre, Inc. |
| E. coli NovaBlue | endA1 hsdR17 (rK12− mK12+) supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proA+B+ lacIqZΔM15::Tn10] (TetR) | Millipore, Inc. |
ORFs b49 and f55 and the predicted origin of replication.
The 1.3-kbp region between the T5 and T6 transcripts harbors two nonconserved ORFs (b49 and f55), the putative origin of replication (42), and several promoters (Fig. 1). ORF b49 is located on the UV-inducible transcript Tind, while ORF f55 is located on the recently discovered transcript Tlys (9, 10, 25). Both of these ORFs could be deleted from SSV1 without a loss of infectivity, although insertion was not tolerated in either ORF (Fig. 1B). Three independent ORF b49 insertion mutants failed to yield infectious virus. Likewise, an ORF f55 insertion mutant was unable to produce infectious virus. Transposon insertions in the intergenic region surrounding these ORFs were also analyzed (Fig. 1B and Table 3). Insertion of the transposon between ORFs b49 and f55 did not inhibit virus infectivity, whereas insertions upstream of ORF f112 and upstream of ORF a100 eliminated infectivity.
The major capsid protein gene, vp1, is essential, but the highly conserved minor capsid gene vp3 is not.
The SSV1 structural genes vp1 and vp3 are highly conserved (Fig. 1A), similar to each other (Fig. 2), and hypothesized to be essential for SSV1 infectivity. As expected, an SSV1 vp1 deletion and an insertion in the middle of the ORF both failed to yield infectious virus (Fig. 1B). In contrast, SSV1 remained infectious following deletion of the universally conserved SSV1 vp3 gene (Fig. 1B). The SSV1-Δvp3 mutant was also shown to be infectious in Sulfolobus solfataricus strains Gϴ, P1, and P2 (Table 4), suggesting this is not a strain-specific phenomenon.
FIG 2.
VP1 and VP3 sequence comparison. Pairwise alignment of the C terminus of SSV1-VP1 (aa 68 to 138) and SSV1-VP3 amino acid sequences. Identical amino acids are highlighted in black, chemically similar amino acids are highlighted in gray, and nonconserved amino acids are not highlighted. Gaps in the alignment are indicated by dashes. The alignment was performed with CLUSTAL-W (59). The 61-bp repeated sequence is indicated with a line under the amino acid sequences.
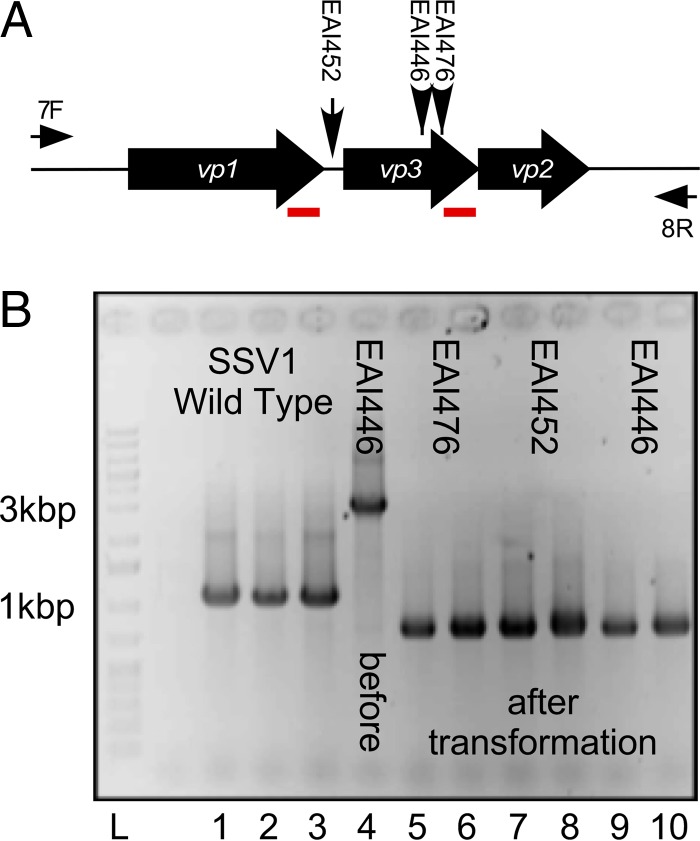
Deletion of the vp3 gene occurs when transposon insertions are present.
Two plasmids with transposon insertions in the SSV1 vp3 gene and one with an insertion in the small intergenic region between SSV1 vp1 and SSV1 vp3 (Fig. 3A) generated infectious virus when transformed into Sulfolobus sp. strain S441. Following transformation, viral DNA was purified from Sulfolobus and screened via PCR using primers that amplified the region encompassing the SSV1 vp1, vp3, and vp2 structural genes (Fig. 3A). Oddly, PCR products generated from DNA isolated from Sulfolobus transformed with the insertion mutants were shorter than those from wild-type SSV1, indicating that a deletion had occurred within this region instead of an insertion (Fig. 3B). DNA sequencing of each PCR product showed that almost the entire vp3 gene was missing. There is an identical 61-bp sequence (7) in the C termini of both the vp1 and vp3 genes (Fig. 2 and 3). Apparently recombination between the 61-bp direct repeats occurred in all of these insertion mutants (Fig. 3). This also resulted in a deletion of the final 15 bp from the vp1 gene, including the stop codon. The new vp1 stop codon is supplied by the native vp3 stop codon (Fig. 2 and 3).
FIG 3.
Insertion mutants in vp3. (A) Overview of vp3 insertion mutants and their analyses. SSV1 structural genes vp1, vp3, and vp2 are indicated as block arrows and labeled in white. The locations of EZ-Tn5 insertion mutants EAI446, EAI452, and EAI476 in the SSV1 vp1/vp3 structural gene region are indicated with vertical arrows. PCR primer (univ_7F and univ_8R) annealing sites are indicated by thin horizontal arrows and labeled 7F and 8R, respectively. Red underlined regions indicate 61-bp direct repeats in the vp1 and vp3 genes. (B) Analysis of vp3 spontaneous deletions. PCR with primers univ_7F and univ_8R was performed on DNA purified from transformed Sulfolobus sp. strain S441 (except lane 4, where DNA was purified from transformed E. coli). Templates: lanes 1 to 3, wild-type SSV1; lane 4, EAI446 DNA purified from E. coli used to transform S441; lanes 5 to 10, DNA from Sulfolobus species transformed with EAI476 (lanes 5 and 6), EAI452 (lanes 7 and 8), and EAI446 (lanes 9 and 10). Lane L, GeneRuler 1-kb plus DNA ladder (Fisher). Relevant molecular masses are indicated beside the gel.
SSV1 virions lacking vp3 are abnormal.
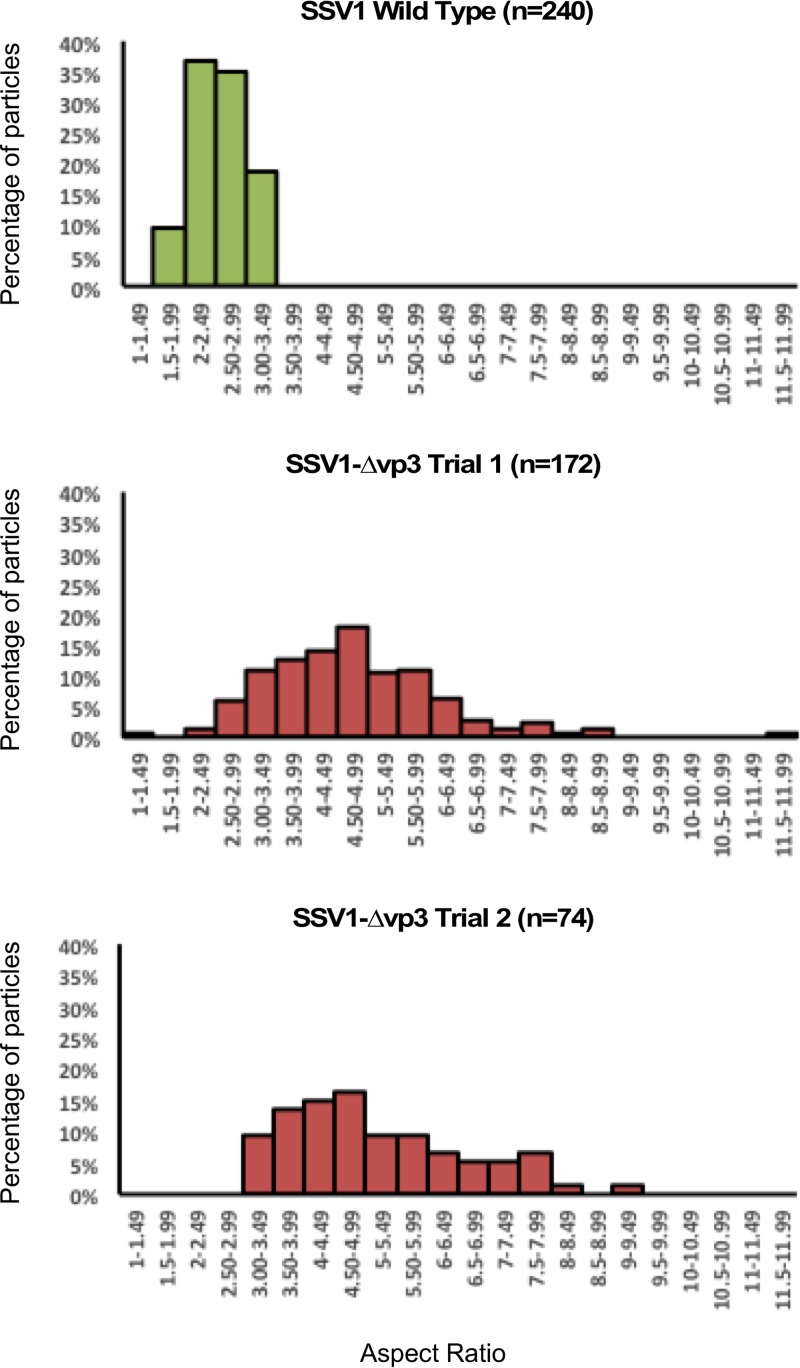
Virions from mutants lacking the vp3 minor capsid protein gene (SSV1-Δvp3), either constructed by LIPCR or generated by recombination, were examined by transmission electron microscopy. While the overall shape of SSV1-Δvp3 virions is that of a spindle, they appeared to be longer and thinner than the wild type (Fig. 4). Length, width, and aspect ratio were measured for 240 wild-type virions stained with uranyl acetate or phosphotungstate. Only 5.4% of wild-type virions were more than 2 standard deviations from the mean of length, width, or aspect ratio. Similarly, 246 SSV1-Δvp3 virions were measured and 99% were more than 2 standard deviations from the means of the wild-type measurements. Moreover, there was much more variability in the shapes of the SSV1-Δvp3 virions than the wild type (Fig. 5).
FIG 5.
Aspect ratios of SSV1 and SSV1-Δvp3 virions. Aspect ratios, defined as the ratio of the long to short axis, of SSV1 wild-type virions (n = 240) and two independent preparations of SSV1-Δvp3 virions (n = 172 and n = 74). Aspect ratios are calculated from measurements of negative-stained TEM images using Image J. Histograms are plotted in bins of 0.5.
DISCUSSION
The SSV1 genome is extremely malleable.
Overall, SSV1 appears to be extremely tolerant of mutagenesis, allowing a variety of insertions and deletions throughout the genome without loss of function (Table 3; Fig. 1B). The extent to which a particular ORF was conserved within the Fuselloviridae generally dictated whether or not the ORF was essential for SSV1 infectivity. Of the 12 ORFs that are unique to SSV1, 8 could be mutated without a loss of infectivity. Conversely, none of the 12 universally conserved genes in the fusellovirus core could be mutated and yet generate infectious virus, with the notable exception of the vp3 gene (Fig. 1). However, two mutants that were initially judged to be noninfectious after 5 negative results, EAI258, a Tn5 insertion in SSV1 ORF c124, and EAI453, a Tn5 insertion between ORFs b49 and f55, were found later to be capable of producing infectious virus (Table 3). Thus, although positive results are definitive, negative results must be considered not to be completely conclusive. Verification of negative results using complementation is ongoing. With a few exceptions, ORFs that could be deleted from the virus genome without loss of infectivity also tolerated insertion of the 2-kb EZ-Tn5 transposon. Many dispensable ORFs encode short, <100-aa, putative proteins. It is not clear whether these ORFs encode proteins or possibly noncoding RNAs. Only products of vp1, vp2, vp3, vp4, and ORF d244 have been reported in purified virions, and no proteomic studies of infected cells have been published (18, 22).
There are fewer essential genes in SSV1 than expected.
We have shown that 16 of the 35 SSV1 ORFs can be disrupted without a loss of infectivity (Fig. 1B). This is a significantly higher number of nonessential genes than previously thought. Insertion of the bacterial plasmid pBluescript into the SSV1 genome following partial endonuclease restriction demonstrated that insertions of pBluescript into SSV1 ORFs e178 and e51 were tolerated, whereas insertions into ORFs e96, b129, vp4, and d335 (integrase) all failed to produce infectious virus (30). These data are in agreement with this work, with the exception of e96, which was shown here to be nonessential by both deletion and insertional mutagenesis (Fig. 1B). Polar effects caused by the insertion of pBluescript could explain this discrepancy, although this seems unlikely, as we have not only isolated a number of infectious insertion mutants in this region but also have shown that this entire quadrant of the SSV1 genome can be deleted without loss of infectivity (Fig. 1B). Alternatively, previous results (30) could have been false negatives.
ORFs encoded by the T5 transcript are almost entirely dispensable.
The T5 transcript is expressed early in the SSV1 transcription cycle after UV irradiation (10) and encodes some of the least conserved fusellovirus ORFs (Fig. 1A). Only three of the 10 ORFs in T5 appear to be essential for infectivity, and two of these (integrase and e54, see below) appear to be essential only in specific hosts (Fig. 1B). Moreover, a deletion mutant lacking ORFs f92, d244, e178, f93, e51, e96, and d63 (∼2.4 kbp) is infectious. This result suggests that SSV1 devotes at least 15% of its genome to ORFs that are seemingly superfluous.
Mycobacteriophage genomes contain a set of well-conserved structural/assembly genes, but much of their genomes are composed of small ORFs (∼500 bp) of unknown function and whose presence varies considerably from isolate to isolate (43). Roughly 2/3 of the nonstructural/assembly genes were not essential for mycobacteriophage function (44), very reminiscent of SSV1 (Fig. 1B). One hypothesis is that these genes were required for growth in an ancestral host or environment but are superfluous under current conditions. Alternatively, some viruses encode genes to compete with cooccurring viruses and/or for protection against host defense systems (45). It is unknown if any fusellovirus genes are involved in any of these putative roles, although many fuselloviruses (not including SSV1) encode a putative Cas4 homologue (46).
The SSV1 integrase gene may be essential in some hosts.
The integrase gene was the first gene deleted from SSV1 (15). The virus lacking the integrase gene was infectious in S. solfataricus strain P2, although the mutant (SSV1-Δint) was quickly outcompeted by wild-type virus (15). However, SSV1-Δint could not infect the Sulfolobus solfataricus strain used in this study (S441). The SSV1-Δint virus was able to infect Sulfolobus solfataricus strain Gϴ, indicating that the SSV1-Δint mutant has a variable host range. Sulfolobus genomes, including that of strain P2, are known to carry a number of integrase-like genes, one of which may be active on a virus lacking an integrase gene (41, 47). Thus, SSV1, contrary to Clore and Stedman (15), does appear to require an integrase gene for infectivity in some host strains. The reason for this requirement remains to be determined.
Deletion of nonconserved ORF e54, which lies just upstream of the integrase start codon, resulted in the same phenotype as the SSV1-Δint mutant. It is possible that deletion of e54 somehow disrupts expression of the integrase gene, effectively resulting in a double mutant. The integrase gene occupies the 3′ end of the T5 transcript and is believed to be transcribed mainly via the T5 promoter. There is evidence that the integrase gene is transcribed from its own promoter. integrase mRNA was found in greater abundance than T5 mRNA following UV induction, and a similar phenomenon was observed for the SSV2 integrase gene during an analysis of the SSV2 transcription cycle (10, 48). Although an obvious promoter has not been identified upstream of the SSV1 integrase gene, several fuselloviruses (SSV2, SSV3, SSV4, and SSV9) encode putative promoters upstream of the integrase gene (49). Thus, based on the low conservation of the e54 ORF and the evidence for an integrase promoter in this region, it seems likely that deletion of e54 simultaneously disrupts expression of the integrase gene and explains the identical phenotype exhibited by both mutants. However, nonconserved ORF f92, which occupies the region of the SSV1 genome where such a promoter would be found, can be deleted without loss of infectivity (Fig. 1).
SSV1 ORF a291 may be a cryptic conserved gene.
The monocistronic transcripts T3 and Tx carry ORFs a291 and c124, respectively, both of which reportedly have homologues in SSV2 (SSV2 ORFs 305 and 126) (50) but were not identified using our BLAST-P analysis. ORF c124 was shown to be nonessential for SSV1 infectivity, while ORF a291 apparently is essential (Fig. 1B). Interestingly, most fusellovirus genomes encode similarly sized ORFs in an identical position just upstream of the putative tail fiber gene (SSV1 vp4), but with little to no detectable overall sequence similarity (5, 6).
Pairwise alignment of SSV1-A291 amino acid sequences and the syntenic SSV2 ORF SSV2-305 revealed that the N-terminal 20 amino acids of the two proteins are highly similar, while the remaining protein exhibits little to no similarity (33). The remaining 10 fusellovirus genomes (Table 1) were reexamined, and nine additional homologues of the N terminus of SSV1-A291 were identified (33). Furthermore, each of these ORFs is preceded by a putative promoter that shares significant similarity with the T3 promoter in SSV1, indicating that transcription of these genes is probably conserved (33). Because at least part of the SSV1 ORF a291 is well conserved in the Fuselloviridae, it is not surprising that it appears essential for SSV1 infectivity. Whether only the N terminus of SSV1-A291 is required is not known.
The fusellovirus core is smaller than expected and is intolerant of mutagenesis.
The fusellovirus core is the set of genes/ORFs that are carried by all known fuselloviruses. By analyzing 11 fusellovirus genomes, the core was reduced to 12 genes/ORFs (Fig. 1A) (5, 6), almost all of which appear to be essential for SSV1 infectivity (Fig. 1B). This is not surprising and reinforces the idea that these are critical to the viral life cycle. The only nonessential core gene identified was SSV1 vp3, which is discussed below. Core fusellovirus ORFs are clustered in one-half of the genome, with the exception of SSV1 ORF b129, and are upregulated during the middle to late portion of the SSV1 transcription cycle (10). Their timing of transcription and coexpression with known structural genes hints that most of the core genes have roles in virus replication, assembly, and packaging, although there is no experimental evidence to support this. We were not able to isolate any functional insertion mutants within this entire half of the genome, even in the poorly conserved and nonessential vp2 gene (11). This insertion could cause a polar effect on the T9 transcript (Fig. 1) but has yet to be tested.
Insertions in ORFs b49 and f55 probably disrupt the SSV1 origin of replication.
Unlike other known fuselloviruses, transcription of SSV1 is strongly induced by UV irradiation and is highly temporally regulated (9, 10). Following UV irradiation, the transcript Tind is immediately upregulated and is swiftly followed by upregulation of the two flanking transcripts, T5 and T6 (9, 10). ORF b49 is the only ORF encoded by Tind and possesses no homology to sequences in public databases, including other members of the Fuselloviridae (Fig. 1A). Due to the abundance of Tind immediately following UV irradiation, it seems likely that the B49 protein plays a role in activation of viral transcription, either directly or indirectly. The b49 ORF can be deleted and apparently is not essential for SSV1 infectivity (Fig. 1B). This agrees well with transcriptomic data from non-UV-induced SSV1-infected cells, where Tind was not detected and presumably not required for infection (25). The effect of the ORF b49 deletion on the SSV1 response to UV irradiation is unknown and could provide insight into this mechanism.
SSV1 encodes a second monocistronic transcript in this region, Tlys, that apparently is expressed constitutively (25, 26). Tlys encodes a 55-amino-acid protein (F55) that is hypothesized to repress transcription from early viral promoters, maintaining low virus expression in the absence of UV irradiation (25). Our results show that ORF f55 is not required for the production of infectious virus. Absence of the F55 protein should result in a loss of repression of early viral promoters that could lead to constant expression of early gene products throughout the infection. However, we have not observed this to date, and plaque morphology appears to be similar to that of the wild type (data not shown).
This area of the SSV1 genome, near Tind and Tlys, contains an abundance of promoters as well as the putative origin of replication and was intolerant of transposon insertion (Fig. 1B). The only functional insertion mutant in this region contained a transposon between the f55 and b49 ORFs, a significant distance from any of the known regulatory elements (Fig. 1B). All other insertions in this region fall within one of the two ORFs or were located adjacent to a promoter. Since ORFs b49 and f55 are not essential, it is unclear why insertions within these ORFs do not produce functional virus. These insertions may disrupt transcription of the T5 or T6 promoter (25, 51), but it is more likely that they disrupt replication. Unpublished data from the Steven D. Bell laboratory has mapped the origin of replication to this region (personal communication). Moreover, GC and purine skew analyses also indicated that the origin is within this area and appears to be well conserved in other fuselloviruses (49). Alternatively, this region could carry essential noncoding RNAs; however, none have been identified to date (10, 50).
The vp3 minor capsid gene is not essential, but mutants have abnormal morphology.
The only nonessential core gene was vp3, which is surprising considering its high degree of conservation and presence as a minor structural protein within the virion (17, 18, 20). Because the VP3 and proteolytically processed VP1 proteins are highly similar (Fig. 2), we hypothesize that VP1 partially complements the SSV1-Δvp3 mutant. SSV1-Δvp3 virions are highly abnormal relative to wild-type SSV1 (Fig. 4 and 5). Elongated particles are often observed in the virions of SSV6, SSV9, and ASV1; however, each of these viruses encodes a VP3 homologue (5, 6). Nonetheless, the dispensability of a seemingly critical gene is unexpected, and the consequences of its loss in regard to virion stability, infectivity, and structure remain to be investigated. Many Sulfolobus genomes encode cryptic fusellovirus genomes/genes (52), thus it is possible that the SSV1-Δvp3 mutant was able to remain infectious via complementation from a host-derived gene product. The sequence of S. solfataricus Gϴ is unavailable, but the S. solfataricus P1 and P2 genomes have been sequenced and do not contain any obvious vp3 homologues, making this scenario unlikely. The abnormal morphology of SSV1-Δvp3 virions is strikingly similar to intermediates in SSV1 budding after UV irradiation (53), indicating that VP3 plays a role in virion maturation.
Insertions in the vp3 minor capsid protein gene are removed.
Following the finding that SSV1-Δvp3 was infectious, several mutants harboring transposon insertions in the vp1-vp3 gene region were isolated and assayed for infectivity (Fig. 1B and 3). Unlike other insertion mutants in the fusellovirus core, insertions in vp3 and the vp1/vp3 intergenic space appeared to be tolerated and did not appear to interfere with the production of infectious virus. However, viral DNA isolated from infected cultures contained a deletion instead of an insertion. The deletion appears to have been facilitated by homologous recombination between two identical 61-bp sequences at the C termini of the vp1 and vp3 genes and results in the nearly complete deletion of the vp3 gene (Fig. 3A). Full-length viral DNA harboring the transposon in this region could not be recovered from infected Sulfolobus cells, indicating that the transposon disrupts SSV1 infectivity and must be eliminated to produce infectious virus; presumably a partially functional VP3 protein or vp3 gene is deleterious. These were the only mutations for which any modification other than LIPCR-mediated deletion or Tn5 insertion was observed.
Our data suggest that this recombination event occurs in Sulfolobus following transformation of transposon-containing mutant DNA and not in E. coli prior to transformation. The same recombination likely occurs in wild-type SSV1, resulting in sporadic loss of the nonessential vp3 gene. However, we have not been able to identify or isolate a spontaneous SSV1-Δvp3 mutant, suggesting that deletion mutants are outcompeted by wild-type virus containing VP3. Thus, an insertion in the vp3 gene is more detrimental to SSV1 than a deletion, but a deletion is less fit than the wild type. SSV1 is the only fusellovirus that possesses such a long stretch of 100% identical bases within its vp1 and vp3 genes. Thus, the phenotype of a vp3 deletion in another fusellovirus would be very interesting.
MATERIALS AND METHODS
Culture conditions.
Infected and uninfected Sulfolobus strains were grown in yeast-sucrose liquid media (YS) and on Gelrite plates at 75°C as described previously (11). E. coli strains were grown on LB media both on agar plates and in liquid cultures with appropriate antibiotics (54). Strains used are listed in Table 4.
Purification of DNA.
Plasmid DNA for LIPCR was purified from transformed E. coli using alkaline lysis (55). Plasmid DNA for transformation into Sulfolobus was purified from E. coli using the GeneJet plasmid purification kit by following the manufacturer's protocols (Thermo-Fisher).
SSV1 DNA used in transposon mutagenesis was purified from previously infected S. solfataricus strain S441 (38). Briefly, 50 ml of SSV1-infected cells was grown for 72 h at 75°C. SSV1 DNA was purified via alkaline lysis followed by 3 phenol-chloroform-isoamyl alcohol (25:24:1) extractions (56). Solvent was removed by passing DNA through a GeneJet plasmid purification column by following the manufacturer's protocol. SSV1 DNA was analyzed by UV absorption spectroscopy (absorption at 260/280 nm of ∼1.8) and endonuclease digestion followed by agarose gel electrophoresis (54).
LIPCR.
LIPCR (15) was used to delete SSV1 ORFs or portions thereof. For deletions, primers were designed to overlap the start and stop codons of the ORF to be deleted. Due to primer design considerations (e.g., incompatible melting temperatures [Tm], unfavorable secondary structures, primer dimers, etc.), most primers include portions of the 5′/3′ ends of the ORFs (Table 5). The optimal concentration of template DNA for LIPCR was determined empirically for each set of primers. Template DNA purified from E. coli with an initial concentration of approximately 200 ng/ml was initially diluted in 30 μl of H2O with 0.01 μg RNase A and further diluted 1:10, 1:50, and 1:100 in double-distilled water (ddH2O). LIPCR was performed as described in Iverson and Stedman (11) using Phusion DNA polymerase. The template for LIPCR reactions was pAJC97 (an SSV1 shuttle vector containing an E. coli plasmid in ORF e178), except for EAI580 and EAI582, which were constructed using a Tn5 insertion in SSV1 as the template. Annealing temperatures for each primer pair were estimated using NEB Tm prediction software (http://tmcalculator.neb.com/#!/) and were experimentally optimized. LIPCR products were purified, phosphorylated, ligated, and transformed into chemically competent NovaBlue E. coli as in Iverson and Stedman (11).
TABLE 5.
Primers used
| LIPCR mutation | Primer sequencea (Tm, °C) |
No. of amino acids remaining in ORF/% of ORF remainingb | |
|---|---|---|---|
| Forward | Reverse | ||
| ΔC102a | CTG AAT GGC TAA AAA GAA CGG (62) | TGT TAT TAT TTC TGT TAC TGA GAC C (57) | 12/11 |
| ΔB251 | ATT GAC GAC GTA ACA AGA TAG (56) | TAA CCA TAA CCA TTC ATT ACT C (55) | 23/9 |
| ΔE54 | ACT CAT TTG TCC ACC TTG (56) | CAA CCA TAA TAC TGT GAG G (53) | 13/24 |
| ΔF92 | CTC TGA GTT GAA TAT CAT TTT CC (58) | ATA GCA TGG CTA GAA TAC AAG G (59) | 16/17 |
| ΔF93 | CAT ATC CTC CTC ACT CCT CAG (60) | ATC AAA AAG AGG TGA ACT AGA TGG (61) | 5/5 |
| ΔE96 | CAG ATC GAA TAT AGG AAC TTG C (59) | TAA ATG ATA GAG AAG AGG AAA GAT AGG (60) | 10/10 |
| ΔF112 | CAT CTT GTA TGA ATT TAG AGT TTG TGC (63) | AAG GCA AAG CAG TGA ACT GAC (63) | 14/13 |
| ΔB49 | GTA GAA GCA ATA AAT GAT TTG (53) | CTC AGA TTT TGC ACA TCC (56) | 15/30 |
| ΔF55 | TTT CCT CGG CAT ACG CTA TC (64) | TAA ATG CCC TAC TAT ACT CTA TCT CTC TC (60) | 4/7 |
| ΔA100 | TTT GAC TTC TGA GGA GG (53) | TAA CTC TTC TTC TTT TCG GG (58) | 15/15 |
| ΔC80 | TTA GCG AGG TAT GTA GAA AAT GTT TAG ATG C (67) | TGT GTA ACA TCT AGG TAA TTT GAT GTA TTC (62) | 23/29 |
| ΔA79 | GTT GAG TGA ATA ATG TAT CAA TGT CTA C (60) | GCA TCT AAA CAT TTT CTA CAT ACC TC (60) | 6/8 |
| ΔA45 | GTG AGA GGA CAA TGA ACC (55) | TTG ATA CAT TAT TCA CTC AAC C (56) | 7/15 |
| ΔC102b | GGA TGA CGG AGT CAG ACG TTG (67) | GTT CAT TGT CCT CTC ACC CTG AAG (67) | 3/3 |
| B129 (ΔN-terminus) | AAA GCG ATT TCA CAG TTT GTC (61) | TGA CTC CGT CAT CCT CTA AC (59) | 64/50 |
| B129 (ΔC-terminus) | AGT TAG GCT CTT TTT AAA GTC TAC C (58) | TGA AAT CGC TTT ACT CGC (59) | 74/57 |
| ΔC124 | AGA AGA TAG CCC TTT TTA AAG CC (62) | GAA AAT AGA ACC TAC AAC TGT AAA CAG (59) | 14/11 |
| ΔB115 | TGG AGG GGT TTA AAA ACG TAA G (63) | TCA TTC CGA CCC CCT AAT TAA C (65) | 33/29 |
| ΔVP1 | TGA GGG ATG GAA ATC AGT TTA AAG (64) | CAA ACT CCT TAG GAG TCT CAT CC (62) | 2/1 |
| ΔVP3 | TGA TAT GAA GTG GGT GCA AAA GG (67) | CAT CCC TCA CAC CTC AGT CTT TG (67) | 2/2 |
| ΔD335 (Integrase) | CAT TTC GCC TCA CAG TAT TAT GG (64) | GTC TGA CAT TAC CCG TAT CAC (58) | 2/1 |
| ΔD63-F92 | TTC TTT ACT CAT TGT TTT TCA CCT TAG (62) | ATA GCA TGG CTA GAA TAC AAG G (59) | NA |
| Other primers | |||
| Univ_7Fc | ATTCAGATTCTGWATWCAGAA | ||
| Univ_8Rd | TCSCCTAACGCACTCATC | ||
Transposon mutagenesis.
The EZ-Tn5 <R6Kγori/KAN-2> insertion kit (Epicentre) was used to perform transposon mutagenesis on purified SSV1 DNA. A molar ratio of 30:1 SSV1 DNA to EZ-Tn5 transposon was found to yield significantly more plasmid constructs containing the entire SSV1 genome than the manufacturer's recommended equimolar ratio (data not shown). This was the only deviation from the manufacturer's protocol (Epicentre). One microliter of the SSV1–EZ-Tn5 reaction was electroporated into 50 μl of Transformax EC100D pir+ electrocompetent E. coli (Epicentre) (Table 4) and plated on LB-agar plates with kanamycin.
Isolation and identification of transposon and deletion mutants.
Plasmid DNA was purified via alkaline lysis (described above) from cultures from single colonies of E. coli following transformation with LIPCR products or transposon insertion reactions. Plasmid DNA was analyzed by restriction endonuclease digestion. All mutations were confirmed by DNA sequencing.
Transformation of Sulfolobus.
Electrocompetent Sulfolobus species cells were prepared from mid-logarithmic cultures (optical density at 600 nm [OD600] of 0.15 to 0.25) by washing with decreasing volumes of 20 mM sucrose essentially as in Schleper et al. (56). The final concentration of cells is ∼1010 cells/ml. One hundred microliters of washed cells was added to a chilled 0.1-cm-gap-length cuvette (Bulldog Bio), and 2 μl of SSV1 DNA (100 to 500 ng/μl) was added to the cells. Cells were transformed by electroporation (Gene Pulser II; Bio-Rad) using the following conditions: 1.5 kV, 400 Ω, 25 μF. Immediately following electroporation, cells were resuspended in 1 ml of 70°C YS, transferred to a 1.5-ml tube, and incubated for 1 h in a 70°C incubator. Following incubation, cells were transferred to 50 ml of preheated YS in a long-neck Erlenmeyer flask and grown with shaking at 70°C. Cultures appeared turbid after ∼24 to 36 h.
Confirmation of infectious SSV DNA.
Spot-on-lawn or halo assays were performed in duplicate 48 and 72 h after transformation of Sulfolobus with SSV DNA. Halo assays were performed as in Iverson and Stedman (11) by spotting 1 to 5 μl of transformed cultures on an indicator lawn of uninfected Sulfolobus on a Gelrite plate, followed by incubation at 70°C for 48 to 72 h. Positive controls included transformation with wild-type SSV1 DNA and known functional mutants. Negative controls were uninfected Sulfolobus cultures. Transformed cultures that inhibited host growth (halo producers) were further analyzed to confirm the identity of the viral DNA. Viral DNA purified from infected cells was amplified with PCR using primers that flank the mutated region of the viral DNA. Control PCRs used the mutant DNA used for transformation and wild-type SSV1 DNA.
Transmission electron microscopy.
For transmission electron microscopy, samples were prepared on 400-mesh carbon-Formvar-coated copper grids (Ted Pella). Grids were placed, carbon-Formvar down, on a 5-μl droplet of culture or culture supernatant for 2 min. Samples were removed from the grid by wicking. Grids were then stained for 60 s on 5 μl of either 2% uranyl acetate stain (pH ∼3) or 2% sodium phosphotungstate tribasic hydrate stain (pH ∼6). Phosphotungstate stain was made freshly every week to ensure that the solution did not disassociate. Grids were allowed to dry in air overnight and were examined within 48 h of staining. Images were obtained at 8,500× to 34,000× magnification on an FEI Tecnai F20 transmission electron microscope (TEM). Grids were analyzed by examining randomly selected grid squares. Images were obtained with a BM UltraScan camera and stored in digital micrograph 3 and TIFF formats.
Particle analysis.
The length and width of images of stained virus particles were measured in ImageJ (58). Normal particle width, length, and aspect ratio were determined using the means of measurements of wild-type SSV1 particles (n = 240). Any particles whose width, length, or aspect ratio was more than two standard deviations from the means were classified as abnormal.
Accession number(s).
Sequences for SSVL and SSV3 have been deposited in GenBank under accession numbers KY563228 and KY579375, respectively.
ACKNOWLEDGMENTS
We thank undergraduate students in the Recombinant DNA Techniques Laboratory at Portland State University (spring terms 2012 and 2013) for initial mapping and activity screening of REC mutants (228-231, 243-245, 262, and 322-325). We also thank Jordan Hartunians for mapping and screening of mutants JAH572, JAH573, and JAH576. We thank C. Martin Lawrence for sharing data on SSV1-B129 and Stephen D. Bell for sharing unpublished data on the SSV1 origin of replication. We thank Valerie P. Huang for isolation of some of the SSV1 EZ-Tn5 insertion mutants.
This work was funded by grants from the National Science Foundation (MCB0702020, MCB1243963, and DMR1263339) and the National Institutes of Health (UL1GM118964) and received support from Portland State University.
REFERENCES
- 1.Prangishvili D. 2013. The wonderful world of archaeal viruses. Annu Rev Microbiol 67:565–585. doi: 10.1146/annurev-micro-092412-155633. [DOI] [PubMed] [Google Scholar]
- 2.Krupovic M, Quemin ERJ, Bamford DH, Forterre P, Prangishvili D. 2014. Unification of the globally distributed spindle-shaped viruses of the Archaea. J Virol 88:2354–2358. doi: 10.1128/JVI.02941-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeats S, McWilliam P, Zillig W. 1982. A plasmid in the archaebacterium Sulfolobus acidocaldarius. EMBO J 1:1035–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stedman KM, She QX, Phan H, Arnold HP, Holz I, Garrett RA, Zillig W. 2003. Relationships between fuselloviruses infecting the extremely thermophilic archaeon Sulfolobus: SSV1 and SSV2. Res Microbiol 154:295–302. doi: 10.1016/S0923-2508(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 5.Redder P, Peng X, Brugger K, Shah SA, Roesch F, Greve B, She QX, Schleper C, Forterre P, Garrett RA, Prangishvili D. 2009. Four newly isolated fuselloviruses from extreme geothermal environments reveal unusual morphologies and a possible interviral recombination mechanism. Environ Microbiol 11:2849–2862. doi: 10.1111/j.1462-2920.2009.02009.x. [DOI] [PubMed] [Google Scholar]
- 6.Wiedenheft B, Stedman K, Roberto F, Willits D, Gleske AK, Zoeller L, Snyder J, Douglas T, Young M. 2004. Comparative genomic analysis of hyperthermophilic archaeal Fuselloviridae viruses. J Virol 78:1954–1961. doi: 10.1128/JVI.78.4.1954-1961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palm P, Schleper C, Grampp B, Yeats S, McWilliam P, Reiter WD, Zillig W. 1991. Complete nucleotide sequence of the virus SSV1 of the archaebacterium Sulfolobus shibatae. Virology 185:242–250. doi: 10.1016/0042-6822(91)90771-3. [DOI] [PubMed] [Google Scholar]
- 8.Nadal M, Mirambeau G, Forterre P, Reiter WD, Duguet M. 1986. Positively supercoiled DNA in a virus-like particle of an archaebacterium. Nature 321:256–258. doi: 10.1038/321256a0. [DOI] [Google Scholar]
- 9.Reiter WD, Palm P, Yeats S, Zillig W. 1987. Gene-expression in Archaebacteria–physical mapping of constitutive and UV-inducible transcripts from the Sulfolobus virus-like particle SSV1. Mol Gen Genet 209:270–275. doi: 10.1007/BF00329653. [DOI] [PubMed] [Google Scholar]
- 10.Fröls S, Gordon PMK, Panlilio MA, Schleper C, Sensen CW. 2007. Elucidating the transcription cycle of the UV-inducible hyperthermophilic archaeal virus SSV1 by DNA microarrays. Virology 365:48–59. doi: 10.1016/j.virol.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Iverson E, Stedman K. 2012. A genetic study of SSV1 the prototypical fusellovirus. Front Microbiol 3:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prangishvili D, Forterre P, Garrett RA. 2006. Viruses of the Archaea: a unifying view. Nat Rev Microbiol 4:837–848. doi: 10.1038/nrmicro1527. [DOI] [PubMed] [Google Scholar]
- 13.Iranzo J, Koonin EV, Prangishvili D, Krupovic M. 2016. Bipartite network analysis of the archaeal virosphere: Evolutionary connections between viruses and capsidless mobile elements. J Virol 90:11043–11055. doi: 10.1128/JVI.01622-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iranzo J, Krupovic M, Koonin EV. 2016. The double-stranded DNA virosphere as a modular hierarchical network of gene sharing. mBio 7:e00978-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clore AJ, Stedman KM. 2007. The SSV1 viral integrase is not essential. Virology 361:103–111. doi: 10.1016/j.virol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence CM, Menon S, Eilers BJ, Bothner B, Khayat R, Douglas T, Young MJ. 2009. Structural and functional studies of archaeal viruses. J Biol Chem 284:12599–12603. doi: 10.1074/jbc.R800078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiter WD, Palm P, Henschen A, Lottspeich F, Zillig W, Grampp B. 1987. Identification and characterization of the genes encoding 3-structural proteins of the Sulfolobus virus-like particle SSV1. Mol Gen Genet 206:144–153. doi: 10.1007/BF00326550. [DOI] [Google Scholar]
- 18.Quemin ERJ, Pietila MK, Oksanen HM, Forterre P, Rijpstra WIC, Schouten S, Bamford DH, Prangishvili D, Krupovic M. 2015. Sulfolobus spindle-shaped virus 1 contains glycosylated capsid proteins, a cellular chromatin protein, and host-derived lipids. J Virol 89:11681–11691. doi: 10.1128/JVI.02270-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Servin-Garcidueñas LE, Peng X, Garrett RA, Martinez-Romero E. 2013. Genome sequence of a novel archaeal fusellovirus assembled from the metagenome of a Mexican hot spring. Genome Announc 1:e0016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon SK, Eilers BJ, Young MJ, Lawrence CM. 2010. The crystal structure of D212 from Sulfolobus spindle-shaped virus Ragged Hills reveals a new member of the PD-(D/E)XK nuclease superfamily. J Virol 84:5890–5897. doi: 10.1128/JVI.01663-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stedman KM, DeYoung M, Saha M, Sherman MB, Morais MC. 2015. Structural insights into the architecture of the hyperthermophilic fusellovirus SSV1. Virology 474:105–109. doi: 10.1016/j.virol.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Menon SK, Maaty WS, Corn GJ, Kwok SC, Eilers BJ, Kraft P, Gillitzer E, Young MJ, Bothner B, Lawrence CM. 2008. Cysteine usage in Sulfolobus spindle-shaped virus 1 and extension to hyperthermophilic viruses in general. Virology 376:270–278. doi: 10.1016/j.virol.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Kraft P, Oeckinghaus A, Kümmel D, Gauss GH, Gilmore J, Wiedenheft B, Young M, Lawrence CM. 2004. Crystal structure of F-93 from Sulfolobus spindle-shaped virus 1, a winged-helix DNA binding protein. J Virol 78:11544–11550. doi: 10.1128/JVI.78.21.11544-11550.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlenker C, Menon S, Lawrence CM, Copié V. 2009. (1)H, (13)C, (15)N backbone and side chain NMR resonance assignments for E73 from Sulfolobus spindle-shaped virus ragged hills, a hyperthermophilic crenarchaeal virus from Yellowstone National Park. Biomol NMR Assign 3:219–222. doi: 10.1007/s12104-009-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fusco S, She QX, Bartolucci S, Contursi P. 2013. T-lys, a newly identified Sulfolobus spindle-shaped virus 1 transcript expressed in the lysogenic state, encodes a DNA-binding protein interacting at the promoters of the early genes. J Virol 87:5926–5936. doi: 10.1128/JVI.00458-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fusco S, She QX, Fiorentino G, Bartolucci S, Contursi P. 2015. Unravelling the role of the F55 regulator in the transition from lysogeny to UV induction of Sulfolobus spindle-shaped virus 1. J Virol 89:6453–6461. doi: 10.1128/JVI.00363-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold HP, She Q, Phan H, Stedman K, Prangishvili D, Holz I, Kristjansson JK, Garrett R, Zillig W. 1999. The genetic element pSSVx of the extremely thermophilic crenarchaeon Sulfolobus is a hybrid between a plasmid and a virus. Mol Microbiol 34:217–226. doi: 10.1046/j.1365-2958.1999.01573.x. [DOI] [PubMed] [Google Scholar]
- 28.Koonin EV. 1992. Archaebacterial virus SSV1 encodes a putative DnaA-like protein. Nucleic Acids Res 20:1143. doi: 10.1093/nar/20.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraft P, Kümmel D, Oeckinghaus A, Gauss GH, Wiedenheft B, Young M, Lawrence CM. 2004. Structure of D-63 from Sulfolobus spindle-shaped virus 1: surface properties of the dimeric four-helix bundle suggest an adaptor protein function. J Virol 78:7438–7442. doi: 10.1128/JVI.78.14.7438-7442.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stedman KM, Schleper C, Rumpf E, Zillig W. 1999. Genetic requirements for the function of the archaeal virus SSV1 in Sulfolobus solfataricus: construction and testing of viral shuttle vectors. Genetics 152:1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirth JF, Snyder JC, Hochstein RA, Ortmann AC, Willits DA, Douglas T, Young MJ. 2011. Development of a genetic system for the archaeal virus Sulfolobus turreted icosahedral virus (STIV). Virology 415:6–11. doi: 10.1016/j.virol.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Jonuscheit M, Martusewitsch E, Stedman KM, Schleper C. 2003. A reporter gene system for the hyperthermophilic archaeon Sulfolobus solfataricus based on a selectable and integrative shuttle vector. Mol Microbiol 48:1241–1252. doi: 10.1046/j.1365-2958.2003.03509.x. [DOI] [PubMed] [Google Scholar]
- 33.Iverson EA. 2015. A genetic and biochemical analysis of Sulfolobus spindle-shaped virus 1. Ph.D. Thesis, Portland State University, Portland, OR. [Google Scholar]
- 34.Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stedman K, Clore A, Combet-Blanc Y. 2006. Biogeographical diversity of archaeal viruses, p 131–143. In Logan NA, Lappin-Scott HM, Oyston PCF (ed), Prokaryotic diversity: mechanisms and significance. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 36.Peng X. 2008. Evidence for the horizontal transfer of an integrase gene from a fusellovirus to a pRN-like plasmid within a single strain of Sulfolobus and the implications for plasmid survival. Microbiology 154:383–391. [DOI] [PubMed] [Google Scholar]
- 37.Muskhelishvili G, Palm P, Zillig W. 1993. SSV1-encoded site-specific recombination system in Sulfolobus shibatae. Mol Gen Genet 237:334–342. [DOI] [PubMed] [Google Scholar]
- 38.Ceballos RM, Marceau CD, Marceau JO, Morris S, Clore AJ, Stedman KM. 2012. Differential virus host-ranges of the Fuselloviridae of hyperthermophilic Archaea: implications for evolution in extreme environments. Front Microbiol 3:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zillig W, Stetter KO, Wunderl S, Schulz W, Priess H, Scholz I. 1980. The Sulfolobus-Caldariella group–taxonomy on the basis of the structure of DNA-dependent RNA-polymerases. Arch Microbiol 125:259–269. [Google Scholar]
- 40.Liu G, She Q, Garrett RA. 2016. Diverse CRISPR-Cas responses and dramatic cellular DNA changes and cell death in pKEF9-conjugated Sulfolobus species. Nucleic Acids Res 44:4233–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.She Q, Singh RK, Confalonieri F, Zivanovic Y, Allard G, Awayez MJ, Chan-Weiher CCY, Clausen IG, Curtis BA, De Moors A, Erauso G, Fletcher C, Gordon PMK, Heikamp-de Jong I, Jeffries AC, Kozera CJ, Medina N, Peng X, Thi-Ngoc HP, Redder P, Schenk ME, Theriault C, Tolstrup N, Charlebois RL, Doolittle WF, Duguet M, Gaasterland T, Garrett RA, Ragan MA, Sensen CW, Van der Oost J. 2001. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc Natl Acad Sci U S A 98:7835–7840. doi: 10.1073/pnas.141222098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannio R, Contursi P, Rossi M, Bartolucci S. 1998. An autonomously replicating transforming vector for Sulfolobus solfataricus. J Bacteriol 180:3237–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendrix RW, Smith MCM, Burns RN, Ford ME, Hatfull GF. 1999. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc Natl Acad Sci U S A 96:2192–2197. doi: 10.1073/pnas.96.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marinelli LJ, Piuri M, Swigonová Z, Balachandran A, Oldfield LM, van Kessel JC, Hatfull GF. 2008. BRED: a simple and powerful tool for constructing mutant and recombinant bacteriophage genomes. PLoS One 3:e3957. doi: 10.1371/journal.pone.0003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatfull GF. 2015. Dark matter of the biosphere: the amazing world of bacteriophage diversity. J Virol 89:8107–8110. doi: 10.1128/JVI.01340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krupovic M, Cvirkaite-Krupovic V, Prangishvili D, Koonin EV. 2015. Evolution of an archaeal virus nucleocapsid protein from the CRISPR-associated Cas4 nuclease. Biol Direct 10:65. doi: 10.1186/s13062-015-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.She Q, Peng X, Zillig W, Garrett RA. 2001. Gene capture in archaeal chromosomes. Nature 409:478. doi: 10.1038/35054138. [DOI] [PubMed] [Google Scholar]
- 48.Ren Y, She Q, Huang L. 2013. Transcriptomic analysis of the SSV2 infection of Sulfolobus solfataricus with and without the integrative plasmid pSSVi. Virology 441:126–134. doi: 10.1016/j.virol.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Clore AJ. 2008. The family Fuselloviridae: diversity and replication of a hyperthermophilic virus infecting the archaeon genus Sulfolobus. Ph.D. thesis, Portland State University, Portland, OR. [Google Scholar]
- 50.Fusco S, Liguori R, Limauro D, Bartolucci S, She QX, Contursi P. 2015. Transcriptome analysis of Sulfolobus solfataricus infected with two related fuselloviruses reveals novel insights into the regulation of CRISPR-Cas system. Biochimie 118:322–332. doi: 10.1016/j.biochi.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Qureshi SA. 2007. Protein-DNA interactions at the Sulfolobus spindle-shaped virus-1 (SSV1) T5 and T6 gene promoters. Can J Microbiol 53:1076–1083. doi: 10.1139/W07-065. [DOI] [PubMed] [Google Scholar]
- 52.Held NL, Whitaker RJ. 2009. Viral biogeography revealed by signatures in Sulfolobus islandicus genomes. Environ Microbiol 11:457–466. doi: 10.1111/j.1462-2920.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 53.Quemin ERJ, Chlanda P, Sachse M, Forterre P, Prangishvili D, Krupovic M. 2016. Eukaryotic-like virus budding in Archaea. mBio 7:e01439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green MR, Sambrook J. 2012. Molecular cloning: a laboratory manual, 4th ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 55.Birnboim HC, Doly J. 1979. Rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schleper C, Kubo K, Zillig W. 1992. The particle SSV1 from the extremely thermophilic archaeon Sulfolobus is a virus–demonstration of infectivity and of transfection with viral-DNA. Proc Natl Acad Sci U S A 89:7645–7649. doi: 10.1073/pnas.89.16.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snyder JC, Spuhler J, Wiedenheft B, Roberto FF, Douglas T, Young MJ. 2004. Effects of culturing on the population structure of a hyperthermophilic virus. Microb Ecol 48:561–566. doi: 10.1007/s00248-004-0246-9. [DOI] [PubMed] [Google Scholar]
- 58.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. [DOI] [PubMed] [Google Scholar]