Abstract
Aim
To report our initial results on the use of radiosurgery for treatment of liver metastases.
Background
In recent years there has been increasing interest in the use of stereotactic body radiation therapy to treat metastatic disease to the liver as an alternative to interventional procedures.
Materials and methods
Between November 2008 and June 2015 a total of 36 LINAC-based radiosurgeries using VMAT were performed in 27 patients with liver metastases from 10 different primary sites. Doses ranged from 21 Gy to 60 Gy in 1 to 5 fractions. In all patients the volume of liver receiving less than 15 Gy was more than 700 cc. The volume treated with the prescription dose ranged from 1 cc to 407 cc with a median of 58 cc. All patients but one received systemic treatment.
Results
Overall median survival for the entire group is 9 months (ranging from 1 to 67 months). Local recurrence free survival ranged from 4 to 67 months with a median of 14 months.
Twenty patients (80%) survived more than six months. Three patients treated for oligometastases were alive after 3 years. Grade 0 toxicity was encountered in 22/27 patients, Grade 1 toxicity in 5/27 and only 1/27 patient experienced Grade 2 toxicity. No patient experienced grade 3–4 toxicity.
Conclusion
Based on these initial results we conclude that SBRT for treating liver metastases with radiosurgery is safe and effective for treating one or multiple lesions as long as normal tissue constraints for liver are respected.
Keywords: SBRT, Liver metastases, Radiosurgery, Oligometastases
1. Background
Many patients with advanced cancer present with liver metastases resulting in substantial morbidity and mortality. Chemotherapy is a standard palliative treatment for most of them, often providing transient responses and increased overall survival. In selected oligometastatic patients, local treatment can lead to long disease-free intervals and even permanent disease control. Surgery remains the gold standard for early metastatic focal disease. However, most patients will not be surgical candidates. For these patients, alternative targeted therapies have been developed. One of these, stereotactic body radiotherapy (SBRT), presents an attractive non-invasive option for selected patients with limited hepatic involvement.1
SBRT is a form of highly precise radiotherapy using high dose of radiation in 5 fractions or less, (extreme hypofractionation) with steep dose distributions tightly covering the tumor, with rapid dose fall off that requires reproducible immobilization, accounting for tumor motion during treatment planning and delivery.2
The liver is a common site for metastases, especially from carcinomas of the colon, lung and breast.3 Liver is the only site of metastatic disease in patients with colorectal cancer in as many as 40% of patients. Fifteen to twenty-five per cent of patients present with liver metastases at the time of diagnosis and this synchronous disease carries the worst prognosis. It is estimated that as many as 55% of patients develop liver metastases during the course of their illness.4
Metastatic spread to the liver is a frequent event in a natural course of many common solid tumors.5, 6, 7 Primary tumor site, histology, extent of liver metastases and the presence of metastatic spread profoundly affect the prognosis.
Stereotactic radiotherapy delivered either as a single fraction or hypofractionated treatment has emerged as a promising alternative to surgical or interventional options in metastatic disease to the liver.8, 9, 10, 11, 12, 13 A treatment scheme focusing on an effective focal radiation is indicated in early phases of disease and after proper patient selection. It can also be used successfully in palliative cases, especially with other metastatic sites present combined with systemic treatment.14
2. Aim
The purpose of this study is to report our initial results on the use of linear accelerator-based radiosurgery as a feasible alternative for the treatment of liver metastases in patients that refuse or are not candidates for surgery.
3. Material and methods
From November 2008 to July 2015, twenty-seven patients with liver metastases were treated with SBRT at one single institution (Fig. 1, Fig. 2, Fig. 3). There were 12 males and 15 females with ages ranging from 42 to 83 years old with a median of 67 and a mean of 66 years. Tumors originated from 10 primary sites. Lung, breast and colorectal cancer were the most common primary sites with 7, 5 and 5 patients, respectively (Table 1). Six patients were oligometastatic, defined as patients who presented with 5 or fewer metastases at any site and with a Karnofsky status (KPS) higher than 70.15, 16
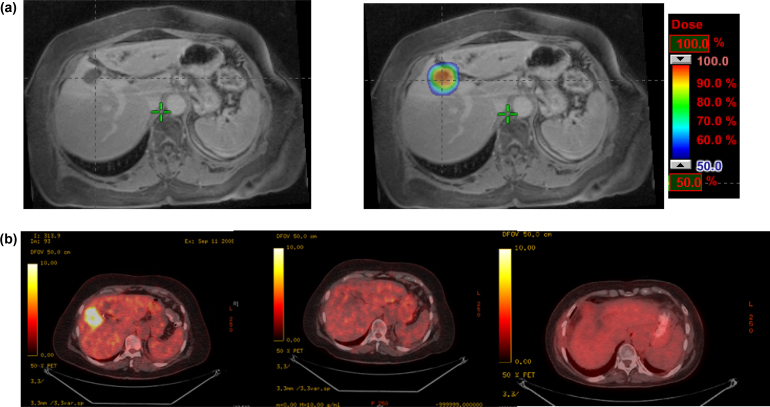
Fig. 1.
72-Year old female with liver metastases from NSCLC. (a) Diagnostic abdomen MRI and dose in color wash in planning treatment using MRI fusion. Dose: integrated boost (12 Gy in the periphery and 15 Gy in the center of the lesion) × 3 fractions. (b) PEC CT at diagnosis and consequently follow-up at 3 months, and 5 years and 11 months after SBRT completion, respectively, showing complete response.
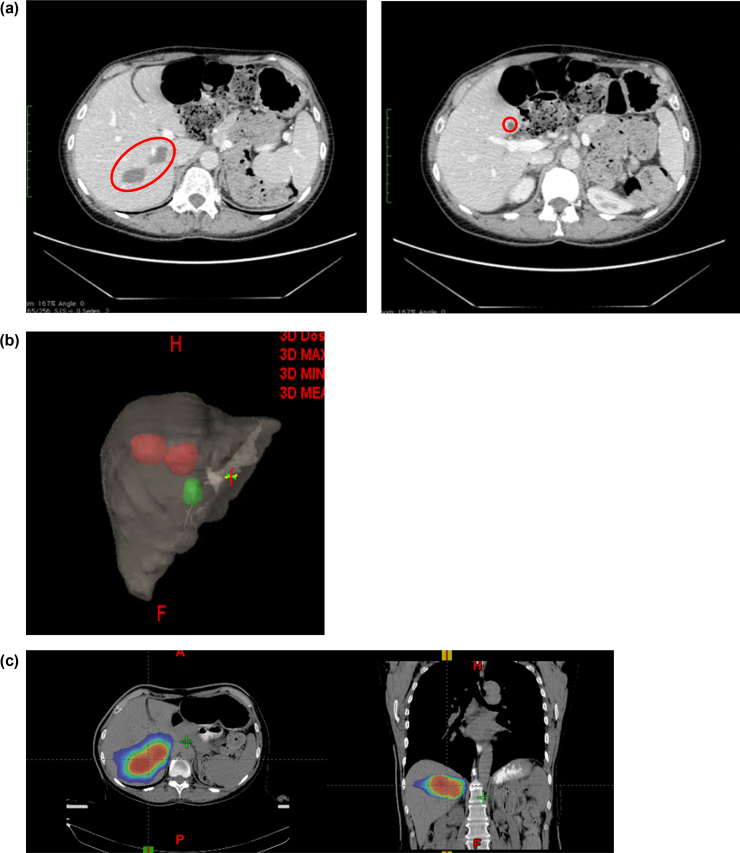
Fig. 2.
62-Year old female with liver metastases from melanoma. (a) Diagnosis CT abdomen showing three lesions. (b) Volumetric image of the liver showing two lesions (in red) treated as a cluster with a single isocenter. Green lesion was treated using a second radiosurgery procedure. (c) First SBRT planning treatment with dose in color wash (15 Gy × 4 fractions). (d) Second single fraction SBRT planning treatment (25 Gy). (e) Dose volume histogram for the composite plan showing volume of liver receiving less than 15 Gy. (f) PEC CT follow-up 5 months after SBRT: excellent response to the therapy. Lesions decreased in size and demonstrated no significant FDG uptake.
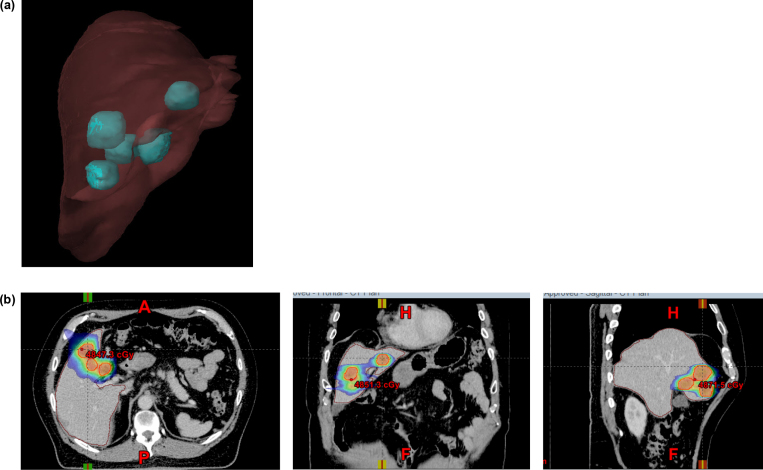
Fig. 3.
64-Year old male with liver metastases from gastric carcinoma. (a) Volumetric image showing the location of 5 liver metastases. (b) Axial, coronal and sagittal views of the isodose distribution in the treatment planning for treating the 5 lesions with single isocenter. Dose: 15 Gy × 3 fractions. Total dose: 45 Gy.
Table 1.
SBRT for liver metastases: a single institution experience.
| Primary tumor | Procedures/n | n alive | OS (median) | Age (median) | Toxicity (acute/late) | Oligomets |
|---|---|---|---|---|---|---|
| Colon | 5/5 | 1 | 22 (2–38) | 74 (42–78) | G0 | 1 |
| Breast | 6/5 | 2 (1LFU)a | 22 (17–38) | 70 (61–72) | Nausea G1 Abd pain G1 |
2 |
| Lung | 12/7 | 1 | 9 (1–67) | 69 (50–83) | Nausea G1 | 2 |
| Endometrium | 1/1 | 0 | 6 | 63 | G0 | 0 |
| Pancreas | 2/2 | 0 | 8 (6–9) | 62 (62–76) | Ascitis G2 | 0 |
| Prostate | 2/1 | 0 | 10 | 73 | G0 | 0 |
| Gallbladder | 1/1 | 1 | 24 | 82 | G0 | 1 |
| Melanoma | 4/2 | 1 (1LFU)a | 5 | 56 (63–49) | Nausea G1 | 0 |
| Ovary | 2/2 | 1 | 7 (6–8) | 54 (43–65) | G0 | 0 |
| Stomach | 1/1 | 1 | 3 | 75 | G0 | 0 |
| Total | 36/27 | 8 | 9 (1–67) | 67 (42–83) | 6 | |
LFU: Lost to follow-up.
3.1. Selection criteria
Selection criteria used were based on tumor location, volume, number of tumors, extent of disease, and general status of the patient. Patients were not surgical candidates due to comorbidities and/or patient refusal to surgery. Criteria for exclusion were active hepatitis or liver failure (encephalopathy, portal hypertension, varices) and presence of clinical ascites.
Treatment intent was curative or palliative including the following:
-
1.
Patients with no evidence of extrahepatic disease with local control of primary tumor or primary tumor potentially controllable.
-
2.
Patients with stable disease.
-
3.
Locally progressive liver disease.
-
4.
Progression of disease after chemotherapy or other previous treatment.
-
5.
Patients with progressive extrahepatic disease.
-
6.
Poor prognosis of the patients due to the presence of one or more liver lesions.
-
7.
Karnofsky performance status (KPS) higher than 70.
-
8.
Sufficient liver volume free of disease to comply with tolerance (more than 700 cc receiving less than 15 Gy).
For pretreatment assessment, all patients underwent a complete physical examination, liver function tests, CT and magnetic resonance imaging (MRI) of the abdomen with intravenous (IV) contrast enhancement unless contraindications were present, as well as whole body PET-CT.
Preplanning and planning steps used to ensure consistent patient position reproducibility included the following steps.
3.2. Pre-treatment
3.2.1. Patient positioning and CT simulation
Abdominal compression to limit diaphragm and intra-fraction intraabdominal organ motion was used for all patients with either compression belt or body thermoplastic masks. In some patients with smaller tumors, CT images from normal respiration, non-extreme exhalation and inhalation were acquired to obtain the internal target volume (ITV) using 1 or 2 mm slices for smaller lesions and up to 2.5 mm for larger lesions. Three phase IV contrast enhanced CT was obtained unless contraindicated.
3.2.2. Treatment planning
Gross tumor volume (GTV) was contoured using planning CT (normal respiration) registered with either MRI or PET-CT diagnostic image. ITV was obtained using images in three respiratory phases. Planning target volume (PTV) margins were usually set at 3–5 mm from ITV depending on the size and location of the lesion as well as per physician discretion. Dose was prescribed depending on the size and location of the lesion with all plans complying with liver tolerance criteria having at least 700 cc of normal liver receiving a dose lower than 15 Gy.
3.2.3. Treatment delivery
Image guided radiation therapy (IGRT) using cone beam CT (CBCT) was used for every fraction. In selected cases we used oral contrast during each treatment fraction to localize the duodenum. Treatment was delivered in 1–5 fractions with doses ranging from 5–20 Gy per fraction. From two to up to 7 coplanar/non coplanar arcs with VMAT Technique (volumetric modulated arc therapy) were used. The most common regimen consisted of 3 fractions of 12 Gy to 20 Gy per fraction. Treatments were not given on consecutive days. Usually two to three fractions per week were delivered and the mean number of elapsed days ranged from 7 to 14. The beam on time ranged from 2 to 9 minutes with a median of 4 and 4 minutes as average.
3.2.4. Follow-up
Every patient was closely followed to evaluate treatment tolerance and acute toxicity during the treatment. First follow-up was at 3 months after completion of treatment. The following ones were each within a 6 months period. Patients were assessed clinically and with serial liver functional tests (LFT) to evaluate late toxicity. Acute and late toxicity was scored according to the Common Terminology Criteria for Adverse Events v4.0 (CTCAE v 4.00). Local failure (LC) was defined by imaging according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Extrahepatic tumor status was classified as either no evidence of disease (NED), stable (SD) or progressive disease (PD). Contrast enhanced CT scans, MRI and/or PET-CT images were used to evaluate the response to treatment.
Besides basic descriptive statistics, Kaplan–Meier statistics was used for survival analysis.
4. Results
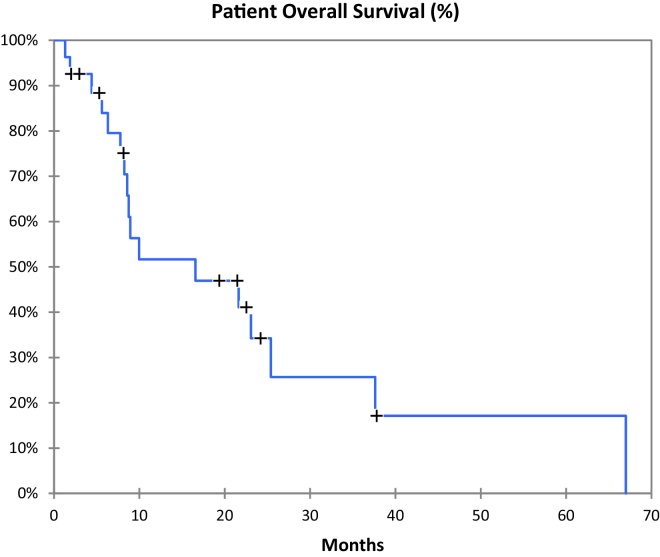
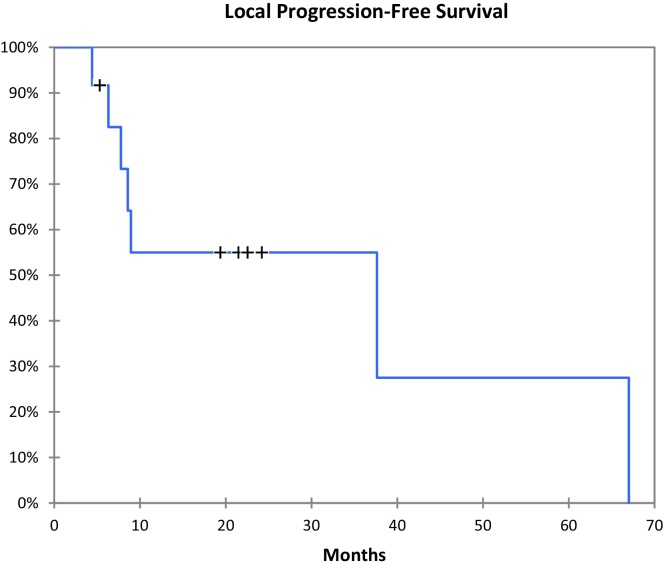
Of the 27 patients treated, two were lost on follow-up. Eight of 25 patients (32%) are alive with a median follow-up of 6 months ranging from 1 to 67 months. Patient overall survival is shown in Fig. 4. Overall median survival after SBRT for the entire group was 9 months (Table 1). Twelve patients had local control (Table 2), eight with progression of extrahepatic disease and four with no evidence of disease. Local progression-free survival (Fig. 5) ranged from 4 to 67 months with a median of 14 months. For this subgroup of twelve patients the total dose delivered ranged from 30 Gy to 60 Gy with a median of 41 Gy and an average of 43 Gy. The minimum and maximum target volumes were 13 cc and 133 cc with a median of 52 cc. Seven patients had local progression. All of them were treated with total dose between 24 Gy and 36 Gy with a median of 27.5 Gy. The minimum and maximum target volumes were 20 cc and 405 cc with a median of 153 cc and average 152 cc. The remainder six patients had stable disease in the liver.
Fig. 4.
Patient overall survival.
Table 2.
Local control vs dose delivery, tumor size and oligometastatic status.
| Liver status | Number of patients | Dose | Tumor size | Oligometastatic |
|---|---|---|---|---|
| LC | 12 | [30–60] 41 Gy | [13–133] 52 cc | 4 O–8 N |
| SD | 6 | [21–60] 34 Gy | [35–271] 97 cc | 1 O–7 N |
| PD | 7 | [24–36] 27 Gy | [20–405] 153 cc | 1 O–6 N |
LC, local control; SD, stable disease; PD, progressive disease.
O, oligometastases; N, non-oligometastases.
Fig. 5.
Local progression-free survival.
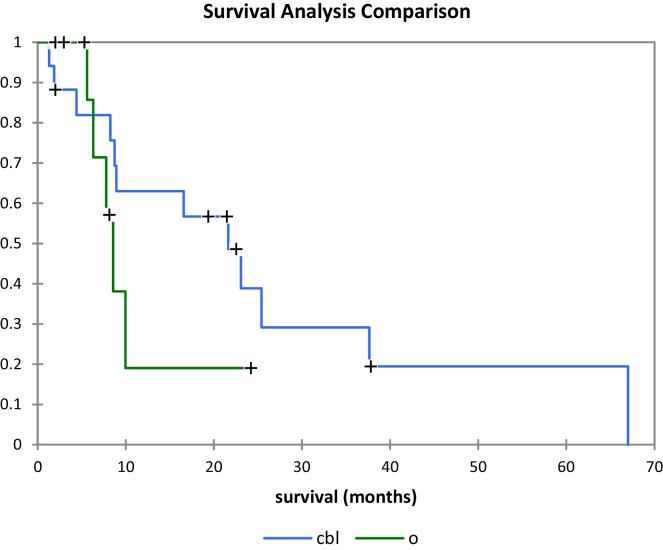
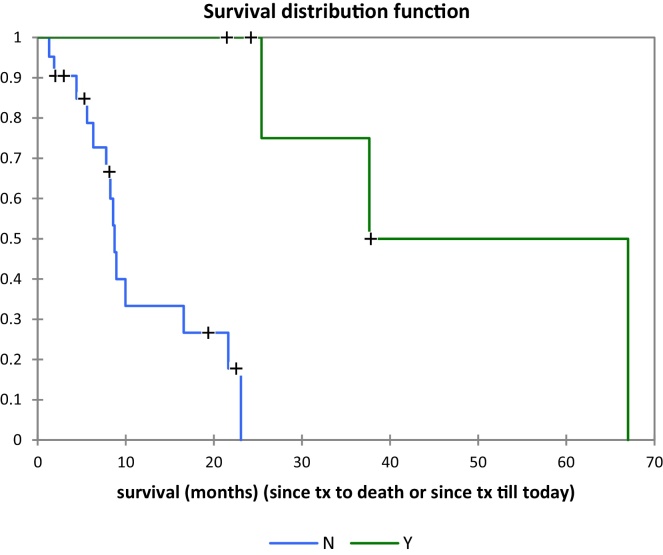
Best survival was achieved in patients with colon, breast and lung primaries (Fig. 6) with median overall survivals of 22, 20 and 9 months, respectively (Table 1). Twenty patients (80%) survived more than six months, ranging from 8 to 67 months with a median of 18 months. Lung, breast, and colorectal cancer were the most common primary sites in this group with 5, 4, and 4 patients, respectively. Ten patients (40%) survived more than 20 months; six of them had oligometastatic disease (2 lung, 2 breast, 1 colon and 1 gallbladder cancer primaries). Three patients (12%) were alive after 3 years. All of them had oligometastatic disease (1 lung, 1 colon, 1 breast). The volumes treated on these three patients were 35 cc, 67 cc and 75 cc, respectively. Survival analysis comparison for oligometastatic patients vs non-oligometastatic patients is shown in Fig. 7.
Fig. 6.
Survival analysis comparison for patient with colon, breast and lung primary (blue) vs other primaries (green).
Fig. 7.
Survival distribution function of oligometastatic (Y green) vs non-oligometastatic (N blue) patients.
One patient with stage IV lung cancer survived 67 months after the first SBRT procedure to the liver and 8 years after being treated to her primary lung cancer (Fig. 1).
Grade 0 toxicity was encountered in 22/27 patients, Grade 1 toxicity in 5/27 and only 1/27 patient experienced Grade 2 toxicity (ascites G2 resolved with paracentesis). No patients experienced grade 3–4 toxicity (Table 3).
Table 3.
SBRT for liver metastases: toxicity grading.
| Toxicity/grade | G1 | G2 | G3 | G4 |
|---|---|---|---|---|
| Abdominal pain | 2 | – | – | – |
| Nausea | 2 | – | – | – |
| Ascite | 1 | 1 | – | – |
| Others | – | – | – | – |
| Total (%) | 5 (18%) | 1 (3%) | 0 | 0 |
Beam on time per fraction ranged from 2 to 9 min with a median of 4 min.
5. Discussion
In selected patients with liver metastases from colorectal cancer consistent with oligometastatic disease such as solitary liver lesions, 5 year survivals in the order of 50% have been reported in highly selected patients.17 In more advanced palliative cases with other metastatic sites present but stable on effective systemic treatment, with so-called oligoprogression, local control of active liver metastases may be necessary.18 In these patients who commonly are not surgical candidates, other potentially curative or palliative alternate less invasive but interventional approaches, including thermal or LASER ablation and chemoembolization, have been utilized. Recently SBRT has become an attractive option for the management of unresectable liver metastases.19
In our institution we started using SBRT for liver metastases in 2008 and most of our patients had rather large or multiple lesions and were not surgical candidates but were in good general condition (Fig. 1, Fig. 2, Fig. 3). The best results were found in patients with oligometastatic disease, with 6 of them surviving after 20 months, and 3 being alive after 3 years. The comparison between the survival distribution functions for oligometastatic and non-oligometastatic patients shows a significant difference with p = 0.002.
A recent experience with SBRT in a rather similar group of patients with heterogeneous primary tumors, and lesion characteristics reflecting a more “real-life” scenario than in many prospective studies was reported by Habermhel et al. with 12- and 18-months local PFS rates of 70% and 59%.20 Our survival results are not as good (local PFS 28% at 20 months) perhaps because of a large percentage of patients (64%) with unfavorable histology (Table 1). In addition, a total dose of less than 36 Gy, used in 12 patients (44%) appears to be related with local failure in our group. Target volume could be another factor influencing results as we see a significant difference between the median and average target volumes (52 cc vs 97 cc vs 153 cc) in the group of patients with local control versus the group of patients that had stable disease and local failure, respectively.
As expected, patients with oligometastatic disease and smaller tumor burden had significantly longer survival.
Although our number of cases is too small for optimal statistical analysis, they tend to confirm previously published data regarding better survival for patients with liver metastases from primary breast and colorectal cancers.
The low toxicity we encountered is likely related to our strict adherence to the previously described protocol to preserve liver function requiring that at least 700 ml of uninvolved liver receive less than 15 Gy in 1–5 fractions. Based on our results, SBRT for treating liver metastases is safe and effective as long as normal tissue constraints for liver are respected (Fig. 2e). Even treating large volumes and multiple lesions mostly in elderly patients, we did not find significant toxicity suggesting that we should not refrain from treating patients who may achieve palliation with a short course of SBRT. This may avoid costly and/or prolonged chemotherapy regimens or other traditional alternatives that may have significant toxicity with lower quality of life.21, 22
6. Conclusions
Based on these initial results we conclude that SBRT for treating liver metastases with radiosurgery is safe and effective for treating one or multiple lesions as long as normal tissue constraints for liver are respected. SBRT for liver metastases can be used in a variety of patients. Dose regimes can vary depending on the intent of the procedure. Better results may be obtained if ablative prescription doses are achieved. However, for symptomatic control lower doses could be advised in order to avoid significant toxicity.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Pocinho R., Roberge D. Stereotactic radiotherapy for liver metastases. Oncol Hematol Rev. 2012;8(1):43–47. [Google Scholar]
- 2.Kavanagh B.D., McGarry R.C., Timmerman R.D. Extracranial radiosurgery (stereotactic body radiation therapy) for oligometastases. Semin Radiat Oncol. 2006;16:77–84. doi: 10.1016/j.semradonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Hoyer M., Swaminath A., Bydder S. Radiotherapy for liver metastases: a review of the evidence. Int J Radiat Oncol Biol Phys. 2012;82(3):1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]; Lawrence T.S., Robertson J.M., Ancher M.S. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 4.Dawood O., Mahadaven A., Goodman K.A. SBRT for liver metastases. Eur J Cancer. 2009;45:2947–2959. doi: 10.1016/j.ejca.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 5.LeGolvan M.P., Resnick M. Pathobiology of colorectal cancer hepatic metastases with an emphasis on prognostic factors. J Surg Oncol. 2010;102:898–908. doi: 10.1002/jso.21817. [DOI] [PubMed] [Google Scholar]
- 6.Kakeji Y., Morita M., Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today. 2010;40:287–294. doi: 10.1007/s00595-009-4152-0. [DOI] [PubMed] [Google Scholar]
- 7.Pagani O., Senkus E., Wood W. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vautravers-Dewas C., Dewas S., Bonodeau F. Image-guided robotic stereotactic body radiation therapy for liver metastases: is there a dose response relationship? Int J Radiat Oncol Biol Phys. 2011;81:e39–e47. doi: 10.1016/j.ijrobp.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 9.Kavanagh B.D., Schefter T.E., Cardenes H.R. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 10.Herfarth K.K., Debus J., Lohr F. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 11.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 12.Lee M.T., Kim J.J., Dinniwell R. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 13.Wulf J., Guckenberger M., Haedinger U. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45:838–847. doi: 10.1080/02841860600904821. [DOI] [PubMed] [Google Scholar]
- 14.Amendola B., Amendola M., Perez N., Blanco J.M., Wu X. Palliative – stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT): innovative and effective tool in the management of advanced cancer using modern radiotherapy instrumentation. J Palliat Care Med. 2015;5:2. [Google Scholar]
- 15.Corbin K.S., Hellman S., Weichselbaum R.R. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol. 2013;31(11):1384–1390. doi: 10.1200/JCO.2012.45.9651. [DOI] [PubMed] [Google Scholar]
- 16.de Vin T., Engels B., Gevaert T., Storme G., De Ridder M. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol. 2013;00:1–5. doi: 10.1093/annonc/mdt537. [DOI] [PubMed] [Google Scholar]
- 17.Isoniemi H., Osterlund P. Surgery combined with oncological treatments in liver metastases from colorectal cancer. Scan J Surg. 2011;100:35–41. doi: 10.1177/145749691110000107. [DOI] [PubMed] [Google Scholar]
- 18.Andratschke N.H.J., Nieder C., Heppt F., Molls M., Zimmermann F. Stereotactic radiation therapy for liver metastatases: factors affecting local control and survival. Radiat Oncol. 2015;10:69. doi: 10.1186/s13014-015-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scorsetti M., Arcangeli S., Tozzi A. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys. 2013;86:336–342. doi: 10.1016/j.ijrobp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Habermehl D., Herfarth K.K., Bermejo J.L. Single-dose radiosurgical treatment for hepatic metastases – therapeutic outcome of 138 treated lesions from a single institution. Radiat Oncol. 2013;8(July):175. doi: 10.1186/1748-717X-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.H. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21(May (17)):5158–5166. doi: 10.3748/wjg.v21.i17.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booth C.M., Nanji S., Wei X. Management and outcomes of colorectal cancer liver metastases in elderly patients: a population-based study. JAMA Oncol. 2015 doi: 10.1001/jamaoncol.2015.2943. [DOI] [PubMed] [Google Scholar]