Figure 6.
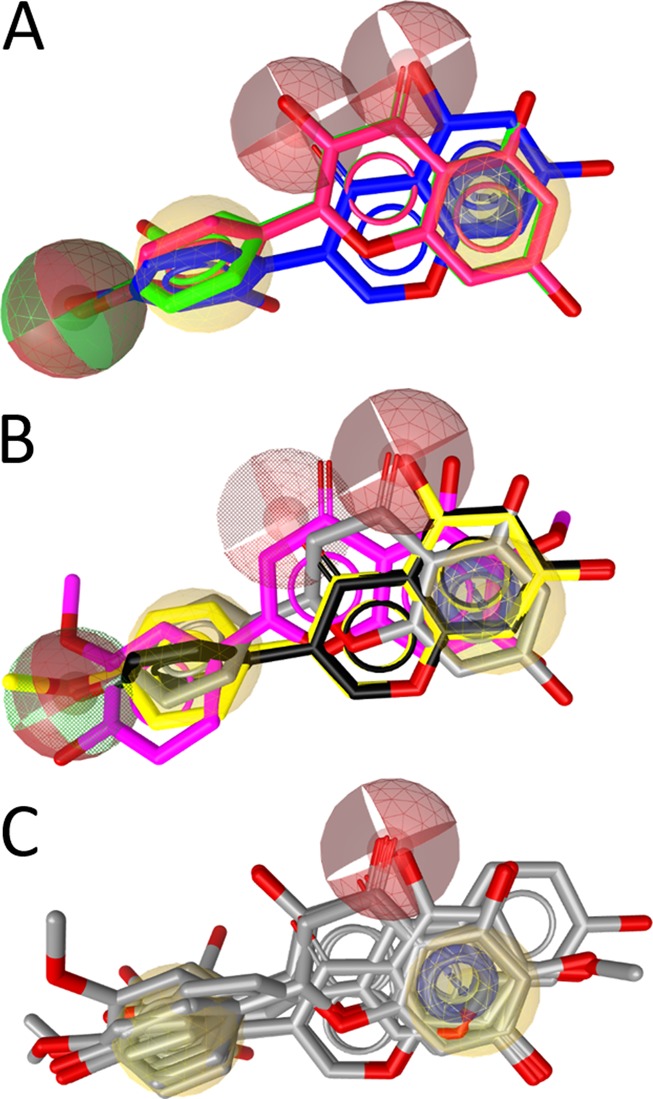
SAR of the flavonoids inhibiting 17β-HSD2. (A) The three most active compounds, 9 (2′-hydroxygenistein, blue), kaempferol (30, red), and quercetin (31, green), share a combined hydrogen bond acceptor/donor at position C-4′, a hydrophobic (aromatic) ring (ring B), two neighboring hydrogen bond acceptors on rings A and C, and the aromatic ring A. (B) The moderately active inhibitors 5 (magenta), naringenin (23, gray), genistein (32, black), and biochanin A (33, yellow) fit into the SAR-pharmacophore illustrating the importance of the hydrogen bond acceptor features on the B and C rings, respectively. (C) For comparison, the general flavonoid model not distinguishing active from inactive compounds is shown with all 17 flavonoids from Table 4.
