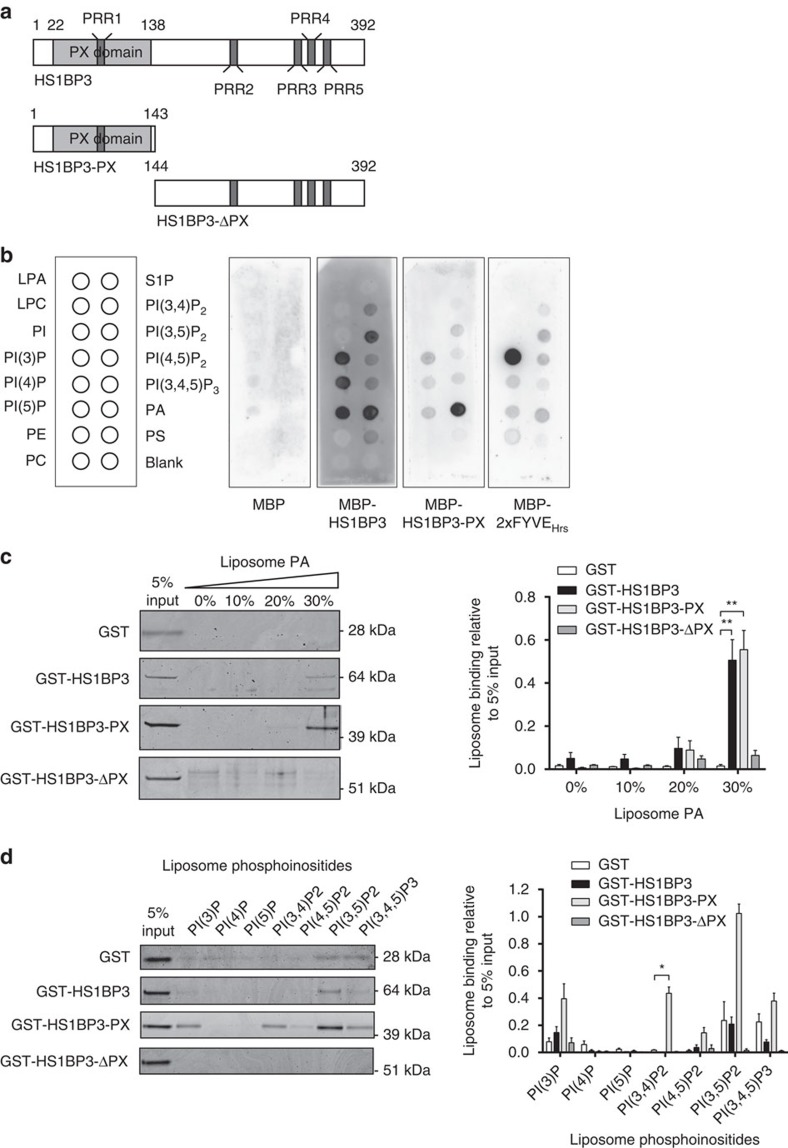
Figure 4. HS1BP3 binds PA through its PX domain.
(a) Domain structure of HS1BP3: an N-terminal PX domain followed by an unstructured C terminus. The positions of five proline-rich regions (PRRs) are indicated. HS1BP3 truncations lacking the C-terminal (HS1BP3-PX) or the PX domain (HS1BP3-ΔPX) are shown. (b) Membranes spotted with the indicated lipids were incubated with 1 μg ml-1 of the indicated recombinant MBP-tagged proteins in a lipid protein overlay assay and bound proteins were detected with anti-MBP immunoblotting. (c,d) Liposomes with the indicated molar ratios of dioleyoyl-phosphatidic acid (DOPA) (c) or the indicated phosphoinositides (d) were incubated with GST or GST-tagged HS1BP3 protein constructs. Protein binding to liposomes was analysed by a lipid floatation assay. Representative coomassie-stained gels are shown and quantified from three independent experiments (mean±s.e.m.). Significance is calculated as compared with GST control. If significance is calculated as compared to GST-HS1BP3-ΔPX, then GST-HS1BP3-PX shows significantly increased binding to PI(3)P, PI(3,4)P2, PI(4,5)P2, PI(3,5)P2 and PI(3,4,5)P3. *P<0.05, **P<0.01, by Student's t-test.