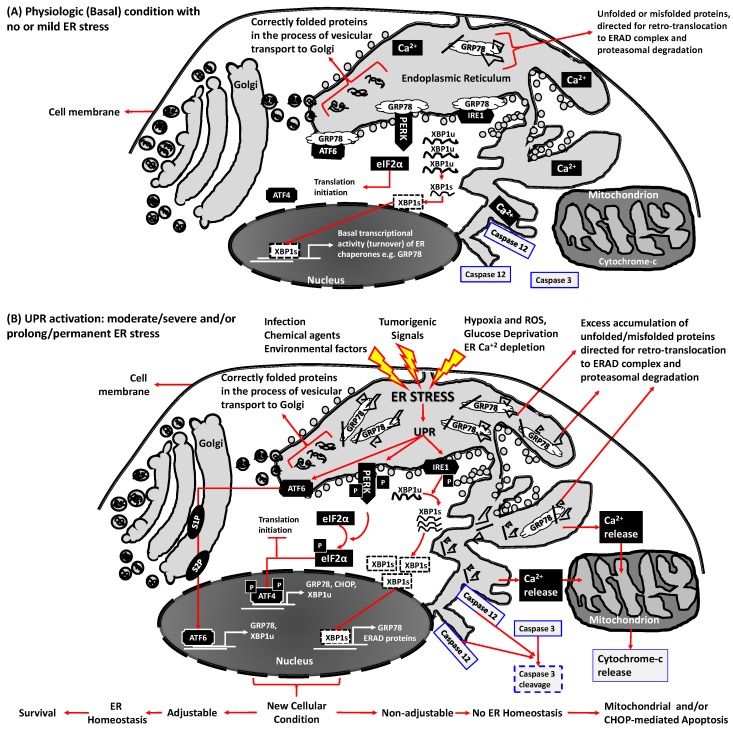
Figure 1.
Endoplasmic Reticulum (ER) homeostasis/stress and the unfolded protein response (UPR) signaling in physiopathologic conditions. The UPR consists of three signaling pathways initiated by detachment of upstream transducers activating transcription factor 6 (ATF6), protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) and inositol-requiring enzyme 1 (IRE1) from glucose-regulated protein 78 (GRP78), a chaperone protein that monitors accumulation of unfolded and misfolded proteins inside the ER lumen. (A) In physiological (unstressed) states, these transducers bind to the folding chaperone GRP78 and keep the ER quiescent; (B) ER stress inducers accumulate unfolded/misfolded proteins in the ER lumen by impairing protein folding. Higher GRP78 affinity for unfolded/misfolded proteins dissociates GRP78 from ATF6, PERK and IRE1, enabling GRP78 unfolded/misfolded protein binding that then initiates three UPR signaling cascades. Specifically: (1) ATF6 signaling involves its translocation to Golgi apparatus for proteolytic cleavage by site-1 protease (S1P) and site-2 protease (S2P) and subsequent release into the nucleus as an active transcription factor to induce expression of GRP78, ubiquitously expressed X-box binding protein 1 (XBP1u) etc.; (2) PERK signaling consist of auto-phosphorylation of PERK (P-PERK), generating an active kinase that phosphorylates eukaryotic translation-initiation factor 2α (P-eIF2α). P-eIF2α blocks its translation initiating activity and induces ATF4 phosphorylation (P-ATF4) leading to P-ATF4 nuclear translocation as a transcription factor to induce expression of GRP78, C/EBP homologous protein (CHOP), XBPu etc.; (3) IRE1α signaling includes IRE1α phosphorylation (P-IRE1α), an active endonuclease that cleaves XBP-1u mRNA to XBP-1s mRNA, which is then translated to an active transcription factor to induce UPR target genes encoding GRP78, ERAD proteins etc. Thus, by increasing ER chaperone protein levels and blocking of eIF2α-mediated protein synthesis, UPR signaling adjusts cells to increased ER stress conditions by transporting excess unfolded/misfolded proteins to ERAD-complex for proteasome-mediated degradation, thereby re-establishing ER homeostasis and sustaining cell survival, whereas prolonged and/or severe ER stress induces apoptosis by CHOP activation, ER-linked caspase 12-mediated caspase 3 cleavage and/or ER Ca2+ efflux associated mitochondrial cytochrome-c release.